
 ŹµŃéŹŅŠčŅŖ0.1mol•L-1NaOHČÜŅŗ480mL£®
ŹµŃéŹŅŠčŅŖ0.1mol•L-1NaOHČÜŅŗ480mL£®·ÖĪö £Ø1£©øł¾ŻŹµŃé²Ł×÷µÄ²½ÖčŅŌ¼°Ćæ²½²Ł×÷ŠčŅŖŅĒĘ÷Č·¶Ø·“Ó¦ĖłŠčŅĒĘ÷£»
£Ø2£©øł¾Żm=nM=cVM¼ĘĖćŠčŅŖĒāŃõ»ÆÄʵÄÖŹĮ棻øł¾Żc=$\frac{n}{V}$·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæ»ņ¶ŌČÜŅŗµÄĢå»żµÄÓ°ĻģÅŠ¶Ļ£®
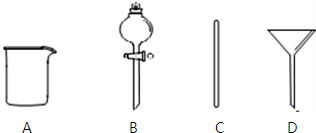
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ²½ÖčĪŖ£ŗ¼ĘĖ攜³ĘĮæ”śČܽā”¢ĄäČ“”śŅĘŅŗ”ś¶ØČŻ”śŅ”ŌČ”ś×°Ęæ”śĢłĒ©£¬Ņ»°ćÓĆĢģĘ½³ĘĮæ£ØÓƵ½Ņ©³×£©³ĘĮ棬ŌŚÉÕ±ÖŠČܽā£¬ĄäČ“ŗó×ŖŅʵ½500mLČŻĮæĘæ£ØŹµŃéŹŅƻӊ480mL¹ęøńµÄČŻĮæĘ棩֊£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬×ŖŅĘĶź±Ļ£¬ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±¼°²£Į§°ō2”«3“Ī²¢½«Ļ“µÓŅŗČ«²æ×ŖŅʵ½ČŻĮæĘæÖŠ£¬ŌŁ¼ÓŹŹĮæÕōĮóĖ®£¬µ±¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬Ź¹ČÜŅŗµÄ°¼ŅŗĆęµÄ×īµĶµćÓėæĢĻßĻąĘ½£¬ČūŗĆĘæČū£¬·“ø“ÉĻĻĀµßµ¹Ņ”ŌČ£®ĖłŅŌŠčŅŖµÄŅĒĘ÷ĪŖ£ŗĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§°ō”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬æĻ¶Ø²»ŠčŅŖ·ÖŅŗĀ©¶·£ØB£©”¢³¤¾±Ā©¶·£ØD£©£¬»¹ŠčŅŖµÄ²£Į§ŅĒĘ÷ŹĒ500mLČŻĮæĘ棬½ŗĶ·µĪ¹Ü£¬
¹Ź“š°øĪŖ£ŗBD£»500mLČŻĮæĘ棬½ŗĶ·µĪ¹Ü£»
£Ø2£©ŹµŃéŹŅƻӊ480mL¹ęøńµÄČŻĮæĘ棬ŠčÅäÖĘ500mL 1mol/LµÄNaOHČÜŅŗ£¬Č»ŗóČ”480mL£¬ŠčŅŖNaOHµÄÖŹĮæ°“500mL¼ĘĖć£¬ÖŹĮæĪŖ£ŗ0.5L”Į0.1mol/L”Į40g/mol=2.0g£¬¶ØČŻŹ±ŃöŹÓČŻĮæĘææĢ¶ČĻߣ¬µ¼ÖĀ¼ÓČėµÄÕōĮóĖ®Ģå»żĘ«“ó£¬ÅäÖʵÄČÜŅŗÅضČĘ«µĶ£¬ŌņĖłµĆČÜŅŗÅØ¶ČŠ”ÓŚ0.1mol•L-1£¬
¹Ź“š°øĪŖ£ŗ2.0£»Š”ÓŚ£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬ŹģĻ¤ŹµŃéŌĄķŗĶŅĒĘ÷µÄŹ¹ÓĆ·½·ØŹĒ½āĢā¹Ų¼ü£¬×¢ŅāÕĘĪÕÅäÖĘ¹ż³ĢÖŠĪó²ī·ÖĪöµÄ·½·ØÓė¼¼ĒÉ£¬ĢāÄæÄŃ¶Č²»“ó£®


 Ņ»æĪŅ»Į·æĪŹ±“ļ±źĻµĮŠ“š°ø
Ņ»æĪŅ»Į·æĪŹ±“ļ±źĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 0.5molH2OµÄ·Ö×ÓŹżĪŖ1.5NA | B£® | 0.5molNaClµÄŌ×ÓŹżĪŖ1 NA | ||
| C£® | 28gCOµÄ·Ö×ÓŹżĪŖ1 NA | D£® | 32gO2µÄŌ×ÓŹżĪŖ1 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH2ØTC£ØCH3£©CH2CH2CH3 | B£® | CH3C£ØCH3£©ØTCHCH2CH3 | ||
| C£® | CH2ØTCHCH£ØCH3£©CH2CH3 | D£® | CH3CH=CHCH£ØCH3£©CH3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ź¹ÓĆČŻĮæĘæĒ°Šč¼ģ²éĖüŹĒ·ńĀ©Ė® | |
| B£® | ½«ÕōĮóĖ®×¢ČėČŻĮæĘæÖŠ£¬ŅŗĆęĄėæĢ¶ČĻßĻĀ1-2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼ÓÖĮŅŗĆęÓėæĢ¶ČĻßĻąĒŠ | |
| C£® | ÅäÖĘČÜŅŗŹ±£¬ÓĆĮæĶ²ĮæČ”ŹŌŃłŗóÖ±½Óµ¹ČėČŻĮæĘæÖŠ£¬»ŗĀż¼ÓČėÕōĮóĖ®ÖĮæĢ¶ČĻß | |
| D£® | ¶ØČŻŗóøĒŗĆĘæČū£¬·“ø“ÉĻĻĀµßµ¹£¬Ņ”ŌČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| “æ¾»Īļ | »ģŗĻĪļ | Ēæµē½āÖŹ | Čõµē½āÖŹ | ·Ēµē½āÖŹ | |
| A | ŃĪĖį | Ė®ĆŗĘų | ĮņĖį | “ĪĀČĖį | øɱł |
| B | ±ł | °±Ė® | Ģ¼ĖįøĘ | Ēā·śĖį | °±Ęų |
| C | ĀČĖ® | ĘÆ°×·Ū | æĮŠŌ¼Ų | ĒāĮņĖį | ĀČ»ÆŅų |
| D | µØ·Æ | ø£¶ūĀķĮÖ | ĀČ»Æ±µ | Ģ¼Ėį | ĀČĘų |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ${\;}_{81}^{203}$TlŗĶ${\;}_{81}^{205}$TlÖŹ×ÓŹżĻąĶ¬ | |
| B£® | ${\;}_{81}^{203}$TlŗĶ${\;}_{81}^{205}$Tl»„ĪŖĶ¬ĖŲŅģŠĪĢå | |
| C£® | ${\;}_{81}^{203}$TlŗĶ${\;}_{81}^{205}$Tl»„ĪŖĶ¬Ī»ĖŲ | |
| D£® | ${\;}_{81}^{203}$TlŗĶ${\;}_{81}^{205}$TlŹĒĮ½ÖÖŗĖĖŲ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com