
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�����Ʊ�ClO2�����������£�
��1��д�����ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��2����ȥClO2�е�NH3��ѡ�õ��Լ��� ��������ĸ��
| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ |
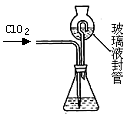
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl�� |
��16�֣�
��1��NH4Cl+2HCl 3H2��+NCl3��2�֣���ƽ��������״̬��ȫ��1�֣�
3H2��+NCl3��2�֣���ƽ��������״̬��ȫ��1�֣�
��2��C��1�֣�
��3�������ղ���Ķ����������壻ʹ��ƿ����ѹǿ��ȣ���1�֣���2�֣�
��2ClO2+10I��+8H+=4H2O+5I2+2Cl����2�֣���ƽ����1�֣�
�����һ�ε���ʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ԭ��2�֣�
��1.35cV��10��2g(2�֣���λ��д��1��)
��4��ʵ�鲽�� ʵ������ ʵ����� ������˿պȡ��ҺX���ھƾ��ƻ��������գ�1�֣� ����ʻ�ɫ��1�֣� ��ҺX�к���Na+ ��ȡ������ҺX����������ϡHNO3��1�֣����ٵμ�����
AgNO3��Һ��1�֣����ְ�ɫ������1�֣� ��ҺX�к���Cl��
���������������1���۲���������ͼ�ɵã���ⲽ��ķ�Ӧ����NH4Cl��HCl����������NCl3��H2�����ݻ��ϼ���������ԭ���غ�ɵã�NH4Cl+2HCl NCl3+3H2������2������������ˮ����������Ҳ������ˮ����ʳ��ˮ�����ܳ�ȥ������Ҳ�ܳ�ȥ�������ȣ���AD��������ʯ�ҵ���Ҫ�ɷ����������ƺ������ƣ��������������ƺ������ƾ�����Ӧ�����Ƕ����������ʯ���ܷ�Ӧ����B����������Ũ�����ܷ�Ӧ����������炙�������泥����������Ȳ�����Ũ���ᷴӦ����C��ȷ����3���ٶ������Ⱦ���ǿ�����ԣ����Խ�KI����ΪI2������Һ��ܿ������ղ���Ķ����������塢ʹ��ƿ����ѹǿ��ȣ���Ϊ��������������ˮ��ƽ����ƿ����ѹ����ʹ������������˳��ͨ�룻�����ڶ������ȡ��������ܽ�KI����ΪI2����������ԭΪ�����ӣ����ݻ��ϼ�����������ɡ�ԭ���غ�ԭ���ɵã�2ClO2+10I��+8H+=2Cl��+5I2+4H2O���۵ζ�ǰ��ƿ����Һ�к���I2�͵��ۣ��������ⵥ�ʱ����������һ�ε���ʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ�Ϊ��ɫ�����ﵽ�ζ��յ㣻������c?V=n�����������ƻ������������ӵ����ʵ���Ϊc��V��10��3mol������I2+2S2O32��=2I��+S4O62����I2��S2O32�������ʵ���֮�ȵ���ϵ��֮�ȿ���I2�����ʵ���Ϊc��V��10��3mol��1/2��Ȼ����2ClO2+10I��+8H+=2Cl��+5I2+4H2O��ClO2��I2�����ʵ���֮�ȵ���ϵ��֮�ȿ���ClO2�����ʵ���Ϊc��V��10��3mol��1/2��2/5�������nM=m����������ȵ�����Ϊc��V��10��3mol��1/2��2/5��67.5g/mol=1.35cV��10��2g����4��NaClO2��NCl3��H2O������Ӧ�����ݻ��ϼ���������ԭ���غ���ƽ�ɵã�6NaClO2+NCl3+3H2O=6ClO2��+NH3��+X�������ơ��ȡ���ԭ�Ӹ����غ�ɵ�XΪ3NaCl��3NaOH������X��Һ����������ʱ������˿պȡ��ҺX���ھƾ��ƻ��������գ�����ʻ�ɫ������X��Һ����������ʱ���������ų����������Ӷ������Ӽ���ĸ��ţ���ȡ������ҺX����������ϡHNO3���ٵμ�����AgNO3��Һ�����ְ�ɫ������˵��X��Һ�к���Cl����
NCl3+3H2������2������������ˮ����������Ҳ������ˮ����ʳ��ˮ�����ܳ�ȥ������Ҳ�ܳ�ȥ�������ȣ���AD��������ʯ�ҵ���Ҫ�ɷ����������ƺ������ƣ��������������ƺ������ƾ�����Ӧ�����Ƕ����������ʯ���ܷ�Ӧ����B����������Ũ�����ܷ�Ӧ����������炙�������泥����������Ȳ�����Ũ���ᷴӦ����C��ȷ����3���ٶ������Ⱦ���ǿ�����ԣ����Խ�KI����ΪI2������Һ��ܿ������ղ���Ķ����������塢ʹ��ƿ����ѹǿ��ȣ���Ϊ��������������ˮ��ƽ����ƿ����ѹ����ʹ������������˳��ͨ�룻�����ڶ������ȡ��������ܽ�KI����ΪI2����������ԭΪ�����ӣ����ݻ��ϼ�����������ɡ�ԭ���غ�ԭ���ɵã�2ClO2+10I��+8H+=2Cl��+5I2+4H2O���۵ζ�ǰ��ƿ����Һ�к���I2�͵��ۣ��������ⵥ�ʱ����������һ�ε���ʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ�Ϊ��ɫ�����ﵽ�ζ��յ㣻������c?V=n�����������ƻ������������ӵ����ʵ���Ϊc��V��10��3mol������I2+2S2O32��=2I��+S4O62����I2��S2O32�������ʵ���֮�ȵ���ϵ��֮�ȿ���I2�����ʵ���Ϊc��V��10��3mol��1/2��Ȼ����2ClO2+10I��+8H+=2Cl��+5I2+4H2O��ClO2��I2�����ʵ���֮�ȵ���ϵ��֮�ȿ���ClO2�����ʵ���Ϊc��V��10��3mol��1/2��2/5�������nM=m����������ȵ�����Ϊc��V��10��3mol��1/2��2/5��67.5g/mol=1.35cV��10��2g����4��NaClO2��NCl3��H2O������Ӧ�����ݻ��ϼ���������ԭ���غ���ƽ�ɵã�6NaClO2+NCl3+3H2O=6ClO2��+NH3��+X�������ơ��ȡ���ԭ�Ӹ����غ�ɵ�XΪ3NaCl��3NaOH������X��Һ����������ʱ������˿պȡ��ҺX���ھƾ��ƻ��������գ�����ʻ�ɫ������X��Һ����������ʱ���������ų����������Ӷ������Ӽ���ĸ��ţ���ȡ������ҺX����������ϡHNO3���ٵμ�����AgNO3��Һ�����ְ�ɫ������˵��X��Һ�к���Cl����
���㣺����̽��ʵ�飬�漰����Ȼ�狀�����Ļ�ѧ����ʽ�������Լ���ѡ���ʹ���װ�õ����á����ӷ���ʽ��������ԭ��Ӧ���ζ��յ���������ʵ���Ũ�ȡ�����Һ����������ʵ������������ӷ���ʽ�е�Ӧ�á����ʵ�鷽������X��Һ�������ӵĴ��ڡ����ʵ�鷽������X��Һ�������ӵĴ��ڵȡ�


 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ̽��ѧϰС��ͬѧ�ú�����������(��ҪΪ������ɳ��CaCl2��MgCl2��Na2SO4��)�Ĵ�����ȡ����ѧ��������NaCl��ʵ��ǰ������������·���(��ͼ)�� 
(1)��д�������ڢܡ��ݲ������Լ����Ƽ��ڢ��������ƣ��� ���� ���� ��
(2) ���������C�Ļ�ѧ�ɷ���(�����ֺͻ�ѧʽ��ʾ)�� ��
(3)д���ڢݲ������п��ܷ�����Ӧ�����ӷ���ʽ��
��
(4)��������������ڢݲ�ʵ���Ƿ�ﵽ��Ŀ�ģ�
��
(5)����Ϊ���������Щ���������Ӱ��ʵ������ ��
(6)��ͬѧ��Ϊ����ʵ����Ʋ�����Լ����������һ�����룺
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С�����ͨп�̷ϸɵ���ڵĺ�ɫ�������̽����������·�����
��֪��I����ͨп�̵�صĺ�ɫ������Ҫ�ɷ�ΪMnO2��NH4Cl��ZnCl2�����ʡ�
II��������пΪ��ɫ��ĩ��������ˮ�������ᡢǿ����Һ�Ͱ�ˮ��
��ش��������⣺
��1���ڲ�����������___________��
��2��ijͬѧ������ҺA�ijɷֺ���NH4Cl��ZnCl2���������һ��ʵ�鷽������֤�������ȷ��Ҫ���ڴ���ϰ��±���ʽд��ʵ�������Ԥ������ͽ��ۡ�
��ѡ�Լ�������ˮ��2moL��L��1 HCI ��2 moL��L��1 HNO3 ��2 moL��L��1 NH3��H2O��6 moL��L��1 NaOH��0.1 moL��L��1 KSCN��0.1 moL��L��1 BaCl2��0.1 moL��L��1 AgNO3����ɫʯ����Һ����ɫʯ����ֽ
| ʵ����� | Ԥ������ | ���� |
| ����1����ȡ������ҺA��װa��b��c��֧�Թܣ���a�Թܣ�__ __________________________ | �а�ɫ�������� | ˵����ҺA����Cl�� |
| ����2����b�Թܣ�__________ __________________________ | ______________________ | _______________________ |
| ����3����c�Թܣ�__________ __________________________ | �Ȳ���_______________, ��____________________ | ˵����ҺA����Zn2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ͷ���һ����������Ʒ����ʳƷ�Ļ�ѧ���ɼ�����С�մ���(̼�����)�������е�����������ɡ�ij�о���ѧϰС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
��1������1����С�մ�ͳ�����ɣ�
����2����С�մ��������ɣ�
����3����________________��ɡ�
�����������̡�
Ϊ̽��ijƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣�ijͬѧ�������ʵ�飬�õ���������
��2���÷��ͷ۵ijɷ�Ϊ________ (�ѧʽ����
��3����һƷ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2������������ʵ����֤��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� |  Ԥ��������� Ԥ��������� |
| 1��ȡ������Ʒ����ϡ�������Һ�ֳ����� | |
| 2�� _______________________________________ | |
| 3�� ________________________________________ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ��������Ĺ������£�
���������գ�
��1������ˮ������������Mg2+��Ca2+�������������A��B������(A��Դ��ʯ��Ҥ�������������B �Ļ�ѧʽΪ ��
��2��ʵ����ģ������Һ�Ʊ�������װ�����£�
��ͼ1��װ�ú�ͼ2��װ�õ����ӷ���Ϊa�� ��b�� ��f��c��
��ͼ2���Լ�ƿ�ڷ����Ļ�ѧ��Ӧ����ʽΪ ��
��ʵ����Ҫ��ͨ���NH3����֮����ͨ��CO2���壬����ͨ���NH3�ѹ�����ʵ������� ��
��3�����������պ�Ĵ����к���δ�ֽ��̼�����ơ�ijͬѧ��ȡ�ô�����Ʒm g���ٳ�ּ������������ٱ仯ʱ�Ƶ�ʣ����������Ϊn g������Ʒ��̼���Ƶ���������Ϊ ��
��4������25���£�0.1mol/LNH3��H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9������˵����ȷ���� ������ţ���
a��0.1mol/L NH4Cl��Һ���Ϻ���Һ�е������ӵ��������Ŀ����ͬ
b����Ϻ����Һ�У�c(NH3��H2O)��c(Cl-)��c(NH4+)��c(OH-)��c(H+)
c���������֪��NH3��H2O�ĵ���̶ȴ���ͬŨ�ȵ�NH4Cl��ˮ��̶�
d�����ǰ������Һ��pH֮�ʹ���14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij����С���һ����̼��ԭ��������ʵ�������IJ������Ũ�����Ȥ����ͨ��ʵ����̽����ɷ֡�
��ʵ��װ�ã�
��һ����̼��ԭ��������ʵ��װ��
��װ��B�з��������ӷ���ʽ��
װ��B��������
��ʵ����������A�еķ�ĩ�ɺ�ɫ��Ϊ��ɫʱ��ֹͣ���ȣ�����ͨһ����̼����ȴ�����£�ֹͣͨ����ͬʱ�۲쵽�����ʯ��ˮ����ǡ�
��ʵ����ۣ�
����Ϊ����������ʵ����������жϳ����ɵĺ�ɫ����Ϊ��������
����Ϊ����������ʵ����������֤�����ɵĺ�ɫ����Ϊ����������������һ��ʵ�飺�ô����������ɵĺ�ɫ���壬�����к�ɫ���屻�������������ǵó����ɵĺ�ɫ����Ϊ�������Ľ��ۡ�
����ͨ���÷�Ӧ��������϶����ǽ��������жϲ�ͨ��ʵ�����������ԣ�
��1����һ�������£�һ����̼���������ڼ��������£��ɷ������·�Ӧ
3Fe2O3+CO 2Fe3O4+CO2
2Fe3O4+CO2
Fe3O4+4CO 4Fe+4CO2
4Fe+4CO2
��2��������������Fe3O4��Ϊ��ɫ���壬��ǿ���ԣ��ܹ�������������
�ס���ͬѧ�Ľ��ۣ� ��Դ����۵������ǣ�
����ʵ��̽��
�Է�Ӧ�����ɷ�������裺
����1����Ӧ�������ֻ��Fe��
����2����Ӧ�������ֻ��Fe3O4��
����3����Ӧ�������_______________________
Ϊȷ��ʵ�������IJ���ijɷ֣���ͬѧ�������ʵ�飬����������ѡ�Լ���������������ɸ�̽�����̣�������д�ڴ����Ӧλ�á�
��ѡ�Լ��������� 1mol/LCuSO4 ��0.01mol/L KSCN��Һ��1mol/L���ᡢ0.01mol/L��ˮ���Թܡ�����������ͷ�ιܡ�
| ʵ����� | Ԥ������ͽ��� |
| ����һ��ȡӲ�ʲ������й�����������ֱ���A��B�Թ��У���������1mol/LCuSO4��Һ�������ܽ⡣ | ��1����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����Ϊ ��2����B�Թ����к�ɫ������������˵����ɫ�����к���Fe�� |
| ����������Թ�B����Һ���ˣ������ù���ϴ�Ӹɾ�������1mol/L����������ηֱ��������0.01mol/L��ˮ������0.01mol/L KSCN��Һ | ��1������Һ�����ɫ���� ��2������Һ���ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���ܴﵽ��ӦĿ�ĵ���
| A����ͼ��װ����ȡ���ռ����� | B����ͼ��װ����ȡ���ռ���ϩ |
| C����ͼ��װ�ý��������ճɻ� | D����ͼ��װ����ȡ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��ʾ����������ʵ�����Ʊ�������ˮFeCl3����������˳����ȷ���ǣ� ��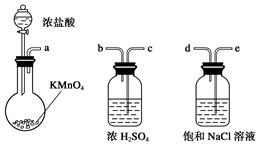
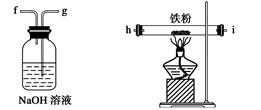
| A��a��b��c��d��e��e��f��g��h |
| B��a��e��d��c��b��h��i��g |
| C��a��d��e��c��b��h��i��g |
| D��a��c��b��d��e��h��i��f |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��ʾ����������ʵ�����Ʊ�������ˮFeCl3����������˳����ȷ����(����)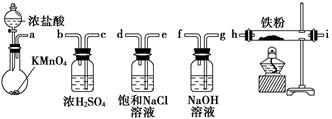
| A��a��b��c��d��e��f��g��h |
| B��a��e��d��c��b��h��i��g |
| C��a��d��e��c��b��h��i��g |
| D��a��c��b��d��e��h��i��f |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com