
��ú��Ϊȼ�Ͽ�ͨ����������;��:
;����C(s)+O2(g) CO2(g)����H1<0��
CO2(g)����H1<0��
;�������Ƴ�ˮú��:
C(s)+H2O(g) CO(g)+H2(g)����H2>0��
CO(g)+H2(g)����H2>0��
��ȼ��ˮú��:
2CO(g)+O2(g) 2CO2(g)����H3<0��
2CO2(g)����H3<0��
2H2(g)+O2(g) 2H2O(g)����H4<0��
2H2O(g)����H4<0��
��ش���������:
(1);����ų���������������������(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ������
(3)��֪:��C(s)+O2(g) CO2(g)����H1="-393.5" kJ��mol-1
CO2(g)����H1="-393.5" kJ��mol-1
��2CO(g)+O2(g) 2CO2(g)����H2="-566" kJ��mol-1
2CO2(g)����H2="-566" kJ��mol-1
��TiO2(s)+2Cl2(g) TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s) TiCl4(s)+2CO(g)�Ħ�H=����������
TiCl4(s)+2CO(g)�Ħ�H=����������
(4)��֪���и����Ȼ�ѧ����ʽ
��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
��3Fe2O3(s)+CO(g) 2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
��Fe3O4(s)+CO(g) 3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
��д��FeO(s)��CO(g)��ԭ��Fe��CO2(g)���Ȼ�ѧ����ʽ��______________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ( ͼ�еġ�H��ʾ����1mol���������)������ͼʾ���ش��������⣺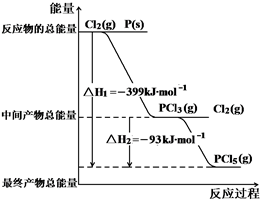
��P��Cl2��Ӧ����PCl3(g)���Ȼ�ѧ����ʽ ��
��PCl5(g)�ֽ��PCl3(g)��Cl2���Ȼ�ѧ����ʽ ��
�ǰ�������ȼ�����ײ�����ȼ������ת��ɺ���Ϊ (���š�������) �ȷ�Ӧ������ð����������Cl2��Ӧ����1molPCl5�ġ�H3�����H3 ��H1 (������������� �� ������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״������������ͼ��ʾ���Իش��������⣺
��1���úϳ�·�߶��ڻ��������ļ�ֵ���� ��
��2��15��20%���Ҵ�����HOCH2CH2NH2��ˮ��Һ���������ԣ������ϳ���·������CO2���ռ��������ӷ���ʽ��ʾ�Ҵ���ˮ��Һ�������Ե�ԭ�� ��
��3��CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5 kJ/mol����H����285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��
2CO2(g) + 6H2(g) CH3OCH3(g) + 3H2O(g)
CH3OCH3(g) + 3H2O(g)
��֪һ��ѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ��±���
| Ͷ�ϱ�[n(H2) / n(CO2)] | 500 K | 600 K | 700 K | 800 K |
| 1.5 | 45% | 33% | 20% | 12% |
| 2.0 | 60% | 43% | 28% | 15% |
| 3.0 | 83% | 62% | 37% | 22% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013�����ȫ�����ض�����ж�������ʮ������������ɡ������족����Ҫ��Դ֮һ������β����ȼúβ���ŷų����Ĺ���С������
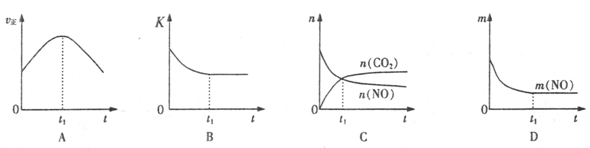
����β����������Ҫԭ��Ϊ��2NO(g)+2CO(g) 2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ� 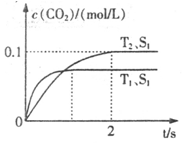
��1���÷�ӦΪ ��Ӧ(����ȡ������ȡ�������T2�¶��£�0~2s�ڵ�ƽ����Ӧ���ʣ�v(N2)= ����2�����������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2���ڴ���ϻ��� c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
��3��ij���л�������t1���£�����㶨���ܱ������У������崫��������˲�ͬʱ���NO��CO��Ũ�ȣ��������ݼ��±���CO2��N2����ʼŨ��Ϊ0����
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c(NO)/xl0-4mol L-1 | 10��0 | 4��50 | 2��50 | 1��50 | 1��00 | 1��00 |
| c(CO)/xl0-3mol L-1 | 3��60 | 3��05 | 2��85 | 2��75 | 2��70 | 2��70 |
 N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪H+(aq)+OH-(aq) H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
H2O(l)����H="-57.3" kJ��mol-1,�ش��������⡣
(1)�ú�20 g NaOH��ϡ��Һ������ϡ���ᷴӦ�ų���������kJ��������
(2)�ú�2 mol H2SO4��ϡ��Һ������ϡNaOH��Ӧ,�˷�Ӧ���к���Ϊ��������������
(3)�����(1)��Ӧ�е�ϡ���ỻ��ϡ����,��Ӧ�ų���������������������(����ڡ���С�ڡ����ڡ�)ԭ��(1)�ų���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ��������Ź㷺��Ӧ�á�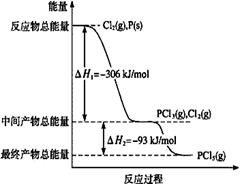
��1������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��ش����⣺
��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ�� ��
��P��Cl2��������Ӧ����1 mol PCl5�ġ�H3�� ��
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0 L�����ܱ������г���1.0 mol PCl5������250 s�ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
| t / s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3) / mol | 0 | 0��16 | 0��19 | 0��20 | 0��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ƻ�1 mol N��N����H��H����N��H���ֱ���Ҫ���յ�����Ϊ946 kJ��436 kJ��391 kJ������1 mol N2(g)��3 mol H2(g)��ȫת��ΪNH3(g)�������仯����ֵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�ٸ�������ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ____________________________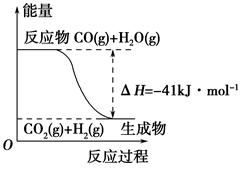
�ڸ�����ͼ��ʾ������ж�����˵������ȷ����______________��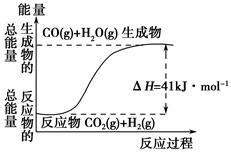
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)=CO2(g)��H2(g)����H��41 kJ��mol��1 |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮
��1�����������У�SO2����������SO3��2SO2��g��+O2��g�� 2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺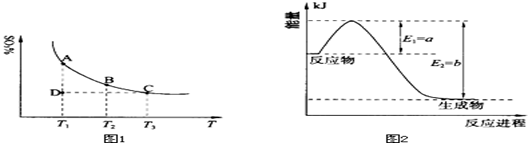
�ٺ��¡���ѹ�����£���Ӧ2SO2��g��+O2��g�� 2SO3��g����ƽ�⣬����ϵ��ͨ�뺤����ƽ�� �ƶ�������������ҡ���������
2SO3��g����ƽ�⣬����ϵ��ͨ�뺤����ƽ�� �ƶ�������������ҡ���������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2�����������������=������ͬ��������Ӧ���е�״̬Dʱ��v�� v�������������������=������ͬ����
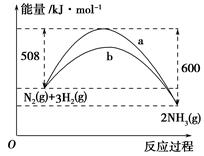
��2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
����ͼ2��һ�����¶Ⱥ�ѹǿ����N2��H2��Ӧ����1molNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ��Ӧ����ʽ�� ��
����H����ֵ�ú���ĸa��b�Ĵ���ʽ��ʾ��
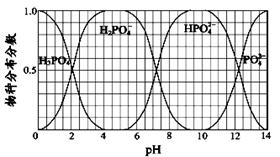
�ڰ�������ˮ�õ���ˮ����25���£���a mol?L-1�İ�ˮ��b mol?L-1������������ϣ���Ӧ����Һǡ�������ԣ��ú�a��b�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ������ʽ ��
��3����֪25��CʱKsp[AgCl]=1.6��10-10mol2?L-2��Ksp[AgI]=1.5��10-16mol2?L-2������25���£���0.1L0.002mol?L-1��NaCl��Һ����μ���0.1L0.002mol?L-1��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�У���������0.1L0.002mol?L-1��NaI ��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com