
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�ü�����ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���жϵζ��յ��������__________________��
��2�����в����п���ʹ����NaOH��Һ��Ũ��ƫ�͵���______��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������������Һ�����Ϊ________mL��
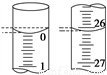
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ����� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.31 | 26.31 |
�ڶ��� | 25.00 | 1.56 | a | 28.74 |
������ | 25.00 | 0.22 | 26.51 | b |
������a����ֵΪ_________�� b����ֵΪ_________��
�����ϱ�������ʽ�����NaOH��Һ�����ʵ���Ũ��Ϊ________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
������ͼװ�ã���ɺܶ�绯ѧʵ�飮�����йش�װ�õ������У���ȷ����
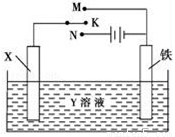
A. ��XΪп����YΪNaCl��Һ������K����M�����ɼ������ĸ�ʴ�����ַ�����Ϊ��������������
B. ��XΪ̼����YΪNaCl��Һ������K����N�����ɼӿ����ĸ�ʴ
C. ��XΪͭ����YΪ����ͭ��Һ������K����M����ͭ�����������ӣ���ʱ���·�еĵ�����ͭ�缫�ƶ�
D. ��XΪͭ����YΪ����ͭ��Һ������K����N�����������������ͭ����Һ��ͭ����Ũ�Ƚ���С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NaOH��Һ����β���е�SO2�������õ�Na2SO3��Һ���е������ѭ��������ԭ����ͼ��a��b���ӽ���Ĥ�����۷ֳ�Ϊ�������缫���Ͼ�Ϊʯī���ס���ֱ���������е�ԭ�ϻ��Ʒ�����б�Ϊ������Һ������˵���������
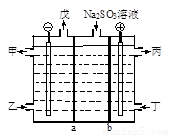
A. ͼ��a��ʾ�����ӽ���Ĥ
B. ����·��ͨ��1mol���ӵĵ���ʱ������0.25mol��O2����
C. ��ΪNaOH��Һ
D. �����ĵ缫��ӦʽΪSO32��+H2O��2e��=SO42��+2H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������Һ�������ɺ����������ʹ������
A. AlCl3 B. Fe2(SO4)3 C. KHCO3 D. NH4HCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и��������ܴ�����������Һ��ɫΪ��ɫ����
A. Na+��MnO4-��K+��NO3-��SO32- B. Na+��S2-��SO32-��H+����NO3-
C. Na+��S2-��OH-��K+��Cl�� D. HCO3-��H+��Na+��Ca2+��SO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NH4Cl��ZnCl2��Һ�������ӽ����еij����
����NaHCO3��Al2(SO4)3������Һ������ĭ����
�۲�ľ�����̬���ʲ��ܻ��ʩ��
��ʵ����ʢ��̼������Һ���Լ�ƿ������ĥ�ڲ�����
�ݼ�������AlCl3��Һ�õ�Al(OH)3����
A. �٢ڢ� B. �ڢۢ� C. �٢ܢ� D. �٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ں����ܱ������У���ͭ��������������������һ������HCl(g)��O2��ȡCl2��ԭ��Ϊ��4HCl(g)��O2(g)  2Cl2(g)��2H2O(g)����H<0�������й�˵������ȷ����
2Cl2(g)��2H2O(g)����H<0�������й�˵������ȷ����
A. ƽ��ǰ�����ŷ�Ӧ�Ľ��У�������ѹǿ��С
B. ƽ��ʱ�������������䣬�����H2O(g)���淴Ӧ���ʼ�С
C. ƽ��ʱ�������������䣬�����¶�ƽ�ⳣ������
D. �����������䣬ʹ�ò�ͬ������HCl(g)��ת���ʲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ʽ���أ�װ�ýṹ��ͼ��ʾ���缫��Ϊ���Ե缫������ʵ������������Һ��ȡ������������ơ�����������ȷ����
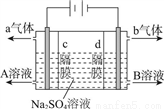
A. a����������b��������
B. AΪ����������Һ��BΪ������Һ
C. ͨ������ʸ��ҵ�SO42��������Ǩ�ƣ���������Һ��pH����
D. �õ�ⷴӦ�ķ���ʽΪ2Na2SO4��6H2O 2H2SO4��4NaOH��O2����2H2��
2H2SO4��4NaOH��O2����2H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ȥ����̼���Ʒ�ĩ�е�����̼�����ƣ�������ķ����ǣ� ��
A. ���� B. ��������������Һ C. �������� D. ����CaCl2��Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com