
£Ø12·Ö£©¼īŹ½Ģ¼ĖįĀĮĆ¾[MgaAlb(OH)c(CO3)d”¤x H2O]³£ÓĆ×÷ĖÜĮĻ×čČ¼¼Į”£
£Ø1£©¼īŹ½Ģ¼ĖįĀĮĆ¾¾ßÓŠ×čČ¼×÷ÓĆ£¬ŹĒÓÉÓŚĘäŹÜČČ·Ö½āŠčĪüŹÕ“óĮæČČĮæŗĶ ”£
£Ø2£©MgaAlb(OH)c(CO3)d”¤x H2OÖŠa”¢b”¢c”¢dµÄ“śŹż¹ŲĻµŹ½ĪŖ ”£
£Ø3£©ĪŖČ·¶Ø¼īŹ½Ģ¼ĖįĀĮĆ¾µÄ×é³É£¬½ųŠŠČēĻĀŹµŃé£ŗ
¢Ł×¼Č·³ĘČ”3.390gѳʷÓė×ćĮæĻ”ŃĪĖį³ä·Ö·“Ó¦£¬Éś³ÉCO20.560L£ØŅŃ»»Ėć³É±ź×¼×“æöĻĀ£©”£¢ŚĮķČ”Ņ»¶ØĮæѳʷŌŚæÕĘųÖŠ¼ÓČČ£¬ŃłĘ·µÄ¹ĢĢ岊ĮōĀŹ£Ø¹ĢĢåѳʷµÄŹ£ÓąÖŹĮæ/¹ĢĢåѳʷµÄĘšŹ¼ÖŹĮæ”Į100%£©ĖęĪĀ¶ČµÄ±ä»ÆČēÓŅĶ¼ĖłŹ¾£ØѳʷŌŚ2700CŹ±ŅŃĶźČ«Ź§Č„½į¾§Ė®£¬6000CŅŌÉĻ²ŠĮō¹ĢĢåĪŖ½šŹōŃõ»ÆĪļµÄ»ģŗĻĪļ£©”£
øł¾ŻŅŌÉĻŹµŃ鏿¾Ż¼ĘĖć¼īŹ½Ģ¼ĖįĀĮĆ¾ŃłĘ·ÖŠµÄn(OH£): n(CO32£)£ØŠ“³ö¼ĘĖć¹ż³Ģ£©”£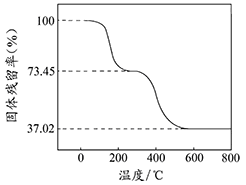
×čÖ¹Č¼ÉÕ
2a+3b=c+2d
£Ø3£©
½āĪöŹŌĢā·ÖĪö£ŗ(1ĪļŹÜČČ·Ö½āĪŖøßČŪµćµÄŃõ»ÆĆ¾ŗĶŃõ»ÆĀĮø²øĒŌŚæÉČ¼Īļ±ķĆę£¬×čÖ¹Č¼ÉÕ£»£Ø2£©ĪļÖŹÖŠø÷ŌŖĖŲµÄŗĻ¼Ū“śŹżŗĶĪŖ0£¬2a+3b=c+2d£»£Ø3£©øł¾Ż·Ö½āĶ¼ĻńŗĶŅŃÖŖŠÅĻ¢£¬µĆ³öµŚŅ»¶ĪŹĒŹ§Č„½į¾§Ė®£¬µŚ¶ž¶ĪŹĒ·Ö½āĪŖŃõ»ÆĪļ£»Ó¦øł¾ŻŗóŅ»¶Ī¼ĘĖćOH-ŗĶCO32-µÄ¹ŲĻµ£¬¼ÓČČŹ±Ē°ÕßÉś³ÉĖ®£¬ŗóÕßÉś³É¶žŃõ»ÆĢ¼£¬¶žÕßµÄŗĶæÉŅŌøł¾ŻĶ¼ÖŠµÄŹż¾Ż»»Ėć£¬ŌŁøł¾Ż¶žŃõ»ÆĢ¼µÄÖŹĮæ¼ĘĖć³öĖ®µÄÖŹĮ棬½ų¶ųµĆµ½OH-ŗĶCO32-µÄĪļÖŹµÄĮ攣
æ¼µć£ŗ±¾Ģāæ¼²é»Æѧ¼ĘĖć”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÖŲ¾§ŹÆæó£ØÖ÷ŅŖ³É·ÖĪŖĮņĖį±µ£©ŅņĪŖŗ¬ÓŠFe2O3”¢MnO¼°ĢæÖŹµČŌÓÖŹ¶ų³£³ŹÉīŗÖÉ«”£¹¤ŅµÉĻ½«ÖŲ¾§ŹÆæó·ŪĖéŗóÓėĮņĖį”¢ĀĮ·ŪŌŚ·“Ó¦²ŪÖŠ»ģŗĻ¼ÓČČ£Ø¼“”°ĘÆ°×”±£©£¬ŌŁ¾Ė®Ļ“µČŅ»ĻµĮŠ¹¤ŠņÖʵư×É«µÄÖŲ¾§ŹÆĢīĮĻ£¬¹ć·ŗÓĆ×÷Ö½ÕÅ”¢ÓĶĘįµČµÄĢī³ä¼Į”£ŅŃÖŖMnOŹĒ¼īŠŌŃõ»ÆĪļ£¬Al·ŪæÉŅŌ½«ÉīÉ«µÄFe3+×Ŗ»ÆĪŖĒ³É«µÄFe2+”£
ÖŲ¾§ŹÆĢīĮĻµÄÉś²ś¹¤ŅÕĮ÷³ĢĪŖ£ŗ
£Ø1£©ŌŚøĆĮ÷³ĢÖŠ£¬ĪŖ¼Óæģ”°ĘÆ°×”±ĖŁ¶Č£¬²ÉČ”µÄ“ėŹ©ÓŠ ”¢
ӣ
£Ø2£©ĮņĖįĘšµ½ĮĖ”°ĘÆ°×”±µÄ×÷ÓĆ”£ĒėŠ“³öĮņĖįÖ±½ÓĘšµ½øĆ×÷ÓĆŹ±µÄ»Æѧ·½³ĢŹ½£ŗ
Ӣ ӣ
£Ø3£©¶ž“ĪĘÆ°×Ė®Ļ“¹żĀĖŗ󣬼ģŃéĀĖŌü²»ŗ¬Fe2+Ąė×ӵķ½·ØŹĒ
Ӣ ӣ
£Ø4£©¶ž“ĪĘÆ°×Ē°£¬ģŃÉÕµÄÖ÷ŅŖÄæµÄŹĒ ”£½«ģŃÉÕŗóµÄ¹ĢĢåÄ„³ÉĻø·Ū£¬Ź¹ÓƵďĒ¼ÓÓŠøÕÓńĒņµÄÕń¶ÆÄ„”£ÕāĖµĆ÷øÕÓń¾ßÓŠŗÜøßµÄ ”£
£Ø5£©¹¤ŅµÉś²śÖŠĪŖĮĖ³ä·ÖĄūÓĆ׏Ō“£¬½«ĀĖŅŗ¾¹ż“¦ĄķµĆµ½»Æ¹¤ŌĮĻFe2O3”£²Ł×÷¹ż³ĢŹĒ£ŗ
¢ŁĀĖŅŗÖŠĶØČė¹żĮæCl2£¬ĘäÄæµÄŹĒ ”£
¢ŚŌŁ¼ÓČėŹŹĮæNaOHĄ“µ÷½ŚČÜŅŗµÄpH £¬ĘäÄæµÄŹĒ £¬µ÷½ŚČÜŅŗµÄpH·¶Ī§ĪŖ ”£
ÓŠ¹ŲĄė×ÓæŖŹ¼³Įµķ¼°ĶźČ«³ĮµķŹ±µÄpHČēĻĀ£ŗ
| Ąė×Ó | æŖŹ¼³ĮµķŹ±µÄpH | ĶźČ«³ĮµķŹ±µÄpH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖijĪļÖŹAÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ 
øł¾ŻÉĻĶ¼¹ŲĻµ¼°ŹµŃéĻÖĻ󣬻Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AŹĒ £¬BŹĒ £¬GŹĒ £¬XŹĒ £Ø¾łĢī»ÆѧŹ½£©”£
£Ø2£©Š“³ö·“Ó¦¢ņµÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©Š“³ö·“Ó¦¢óµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ŅŃÖŖA”«GÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·ÖÉś³ÉĪļŅŃĀŌČ„£©£¬ĘäÖŠA”¢GĪŖµ„ÖŹ£¬DŹĒÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶É«µÄĘųĢ壬E”¢F¾łÄÜÓėNaOHČÜŅŗ·“Ó¦”£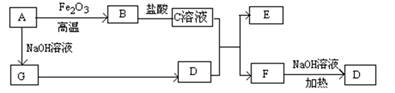
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öFµÄµē×ÓŹ½ £»
£Ø2£©¢ŁCČÜŅŗÓėD·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»
¢ŚFČÜŅŗÓėNaOHČÜ???¹²ČČ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
£Ø3£©¢ŁĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶCČÜŅŗĪŖŗĪĻŌĖįŠŌ £»
¢ŚFČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ £»
£Ø4£©½«5.4gAĶ¶Čė200mL 2.0mol/LijČÜŅŗÖŠÓŠGµ„ÖŹ²śÉś£¬ĒŅ³ä·Ö·“Ó¦ŗóÓŠ½šŹōŹ£Óą£¬ŌņøĆČÜŅŗæÉÄÜŹĒ £ØĢī“śŗÅ£©
A£®HNO3ČÜŅŗ B£®H2SO4ČÜŅŗ C£®NaOHČÜŅŗ D£®HClČÜŅŗ
£Ø5£©½«1molN2ŗĶ3molG¼°“߻ƼĮ³äČėČŻ»żĪŖ2LµÄijĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£¬ŅŃÖŖøĆ·“Ó¦ĪŖ·ÅČČ·“Ó¦”£Ę½ŗāŹ±£¬²āµĆDµÄĪļÖŹµÄĮæÅضČĪŖa mol/L”£
¢ŁČē¹ū·“Ó¦ĖŁĀŹv(G)£½1.2mol/(L”¤min)£¬Ōņv(D)£½ mol/(L”¤min)
¢ŚŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ČōĘšŹ¼Ź±³äČė0.5molN2ŗĶ1.5molG“ļµ½Ę½ŗāŗó£¬DµÄĪļÖŹµÄĮæÅØ¶Č £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©a/2 mol/L”£
¢ŪøĆĢõ¼žĻĀµÄĘ½ŗā³£ŹżĪŖ £ØÓĆŗ¬aµÄ“śŹżŹ½±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ijŅŃÖŖA”¢B¾łŹĒÓÉĮ½ÖÖ¶ĢÖÜĘŚŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ£¬A֊ijŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ25%£¬BµÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬C”¢J”¢XŹĒĶ¬ÖÜĘŚµÄŌŖĖŲµÄ¼ņµ„Ēā»ÆĪļ£¬XĪŖĪŽÉ«ŅŗĢ壬C”¢JĪŖĘųĢ壬DŹĒŅ»ÖÖ²»ČÜÓŚĖ®µÄ°×É«¹ĢĢ唣·“Ӧɜ³ÉµÄĖ®¾łŅŃĀŌČ„”£ĖüĆĒÓŠČēĻĀĶ¼ĖłŹ¾µÄ¹ŲĻµ”£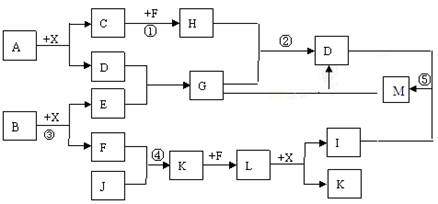
£Ø1£©Š“³ö»ÆѧŹ½£ŗA_________ E___________ L___________£»
£Ø2£©ŌŚ·“Ó¦¢Ł¢Ś¢Ū¢Ü¢ŻÖŠŹōÓŚŃõ»Æ»¹Ō·“Ó¦µÄŹĒ_____________£»
£Ø3£©·“Ó¦¢Ū»Æѧ·½³ĢŹ½ĪŖ£ŗ______________________________£»
£Ø4£©Š“³öĻĀĮŠĄė×Ó·½³ĢŹ½£ŗ·“Ó¦¢Ś £»GČÜŅŗÓėMČÜŅŗµÄ·“Ó¦___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
×ܵÄĪļÖŹµÄĮæĪŖ0.50 molµÄFe·ŪÓėAl·Ū»ģŗĻĪļ·ÖĪŖµČÖŹĮæµÄĮ½·Ż£»ŌŚŅ»·ŻÖŠ¼ÓČė×ćĮæ
µÄĻ”ŃĪĖį£¬ŌŚ±ź×¼×“æöĻĀ²śÉśĘųĢåa L£»ŌŚĮķŅ»·ŻÖŠ¼ÓČė×ćĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ŌŚ±ź×¼×“æöĻĀ²śÉśĘųĢåb L”£Ōņa+bµÄŹżÖµ²»æÉÄÜŹĒ
| A£®5.6 | B£®7.3 | C£®8.2 | D£®11.2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
½«70 g¹żŃõ»ÆÄĘŗĶŃõ»ÆÄʵĻģŗĻĪļøś98 gĖ®³ä·Ö·“Ó¦ŗó£¬ĖłµĆĒāŃõ»ÆÄĘČÜŅŗµÄÖŹĮæ·ÖŹżĪŖ50%”£
£Ø1£©ĒóŌ»ģŗĻĪļÖŠ¹żŃõ»ÆÄĘŗĶŃõ»ÆÄʵÄÖŹĮ棻£Ø2£©²śÉśµÄĘųĢå±źæöĻĀĢå»ż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ä³Ń§ÉśÓĆŹµŃéŹŅ³£¼ūµÄĖį”¢¼ī”¢ŃĪŗĶ½šŹōµ„ÖŹĪŖ·“Ó¦Īļ£¬²¢ĄūÓĆŅ»øöµ×²æÓŠŠ”æ׵ďŌ¹ÜŗĶŅ»øö¹ćæŚĘæ×é×°³ÉČēĶ¼ĖłŹ¾µÄ×°ÖĆ”£ŹŌ»Ų“š£ŗ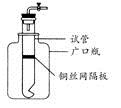
(1)ČōŹŌ¹Ü֊װӊĶĖæĶųøō°å£¬ĄūÓĆøĆ×°ÖĆæÉÖĘČ”ÄÄŠ©ĘųĢå£æ
(Š“³öĮ½ÖÖ)”£
(2)Čō½«ĶĖæĶųøō°åøÄĪŖĢśĖæĶųøō°å£¬ŌņøĆ×°ÖĆæÉÓĆÓŚÖĘČ”ŗĪÖÖĘųĢå£æ
ӣ
øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ÓĆŗ¬ÓŠAl2O3”¢SiO2ŗĶÉŁĮæFeO”¤xFe2O3µÄĀĮ»ŅÖʱøAl2(SO4)3”¤18H2O£¬¹¤ŅÕĮ÷³ĢČēĻĀ(²æ·Ö²Ł×÷ŗĶĢõ¼žĀŌ)£ŗ
¢ń.ĻņĀĮ»ŅÖŠ¼ÓČė¹żĮæĻ”H2SO4£¬¹żĀĖ£»
¢ņ.ĻņĀĖŅŗÖŠ¼ÓČė¹żĮæKMnO4ČÜŅŗ£¬µ÷½ŚČÜŅŗµÄpHŌ¼ĪŖ3£»
¢ó.¼ÓČČ£¬²śÉś“óĮæ×ŲÉ«³Įµķ£¬¾²ÖĆ£¬ÉĻ²ćČÜŅŗ³Ź×ĻŗģÉ«£»
¢ō.¼ÓČėMnSO4ÖĮ×ĻŗģÉ«ĻūŹ§£¬¹żĀĖ£»
¢õ.ÅØĖõ”¢½į¾§”¢·ÖĄė£¬µĆµ½²śĘ·”£
£Ø1£©H2SO4ČܽāAl2O3µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________________”£
£Ø2£©½«MnO4-Ńõ»ÆFe2£«µÄĄė×Ó·½³ĢŹ½²¹³äĶźÕū£ŗ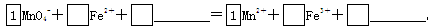
£Ø3£©ŅŃÖŖ£ŗ
Éś³ÉĒāŃõ»ÆĪļ³ĮµķµÄpH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| æŖŹ¼³ĮµķŹ± | 3.4 | 6.3 | 1.5 |
| ĶźČ«³ĮµķŹ± | 4.7 | 8.3 | 2.8 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com