
NaClO2��һ����Ҫ��ɱ������������һ����������ԭ�����£�
��2NaClO3+SO2+H2SO4==2ClO2+2NaHSO4
��2ClO2+NaCl 2NaClO2+Cl2
2NaClO2+Cl2
�ش��������⣺
��1��NaClO2�е�Cl �Ļ��ϼ�__________��
��2������Ӧ�ٸ�Ϊ���ӷ�Ӧ����ʽ_______________________��
��3�������Ӧ�ڵĵ���ת�Ƶķ������Ŀ_________________________��
��4�����ڵ���ʳ��ˮ���ȳ�ȥ����Ca2+��Mg2+��SO42-�����ʣ����Ӳ���ʱ�����μ�����Լ�˳��ΪNaOH��_________��_________��ַ�Ӧ������һ�����˳�ȥ��
��5����������β����������ClO2��(�������Ⱦ���ǿ������)������H2O2������Һ���ճ�ȥ���÷�Ӧ�е���������Ϊ_________��
��6��KClO2��Cl2���ܽ���Ʒ�ˮ��CN- ����Ϊ�������ʣ���������ԭΪCl-��������CN- ��ͬ����Ʒ�ˮ����Cl2�����ʵ�����KClO2__________����
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ĸ߶����¿�����ѧ���������棩 ���ͣ�ѡ����
������Һ������Ũ�ȹ�ϵ �ı�ʾ��ȷ����( )
�ı�ʾ��ȷ����( )
A��NaHCO3��Һ�У�c(H��)��c(Na��)��c(OH��)��c(CO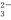 )��c(
)��c( HCO
HCO )
)
B��pH��3��CH3COOH��Һ��pH��11��NaOH��Һ�������Ϻ����Һ�У�c(OH��)>c(H��)��c(CH3COO��)
C��0.1 mol��L��1��NH4Cl��Һ�У�c(Cl��)>c(H��)>c(NH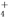 )>c(OH��)
)>c(OH��)
D�����ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������Ϻ����Һ�У�
2c(Na��)��c(CH3COOH)��c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ĸ�һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���������� Һ
Һ : ��KNO3��NaCl��Һ ��H2O��C2H5OH(�Ҵ�) ��Br2��CCl4��Һ�����ϸ����Һ�ķ��뷽��������
: ��KNO3��NaCl��Һ ��H2O��C2H5OH(�Ҵ�) ��Br2��CCl4��Һ�����ϸ����Һ�ķ��뷽��������
A����Һ��������ȡ B���ᾧ��������ȡ
C���ᾧ���������� D�����ˡ�������ȡ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꼪��ʡ��һ���¿�����ѧ���������棩 ���ͣ�ѡ����
ij��Һ��������Ӧ�ų������������Һ�п϶����ܴ����������������
A��NH4+��Na����Ba2����Cl�� B��Na����I����HCO3����SO42��
C��K����Cl����SO32����AlO2�� D��Na����Al3����SO42����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꼪��ʡ��һ���¿�����ѧ���������棩 ���ͣ�ѡ����
��֤���������������̼�����Ե�ʵ����ʵ��
A��CO2����ˮ�γ�̼�ᣬSiO2������ˮ
B��CO2ͨ������Թ������������������
C��������SiO2��̼���η�Ӧ����CO2
D���Ȼ���ͨ�������̼������Һ�зų����壬ͨ������Թ�������Һ�����ɳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
������������ȷ����
A. �������Ż�ʱ����ϸɳ�������
B. ��100mL��Ͳ������0.1000 mol��L-1Na2CO3��Һ
C. ��ȼ�Լ���ǿ�������Լ��ֿ����ò�Զ���Դ
D. Ũ�����P2O5���Ը���Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
������Һ�У�һ���ܴ����������������
A��K+��Ba2+��SO42-��HCO3- B��Al3+��Fe2+��Cl-��MnO4-
C��Na+��NH4+��NO3-��Cl- D��K+��Mg2+��NO3-��OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
�������з�Ӧ���κ��¶��¾����Է����е���
A��2N2(g)��O2(g)=2N2O(g) ��H����163 kJ��mol��1
B��Ag(s)��1/2Cl2(g)=AgCl(s) ��H����127 kJ��mol��1
C��HgO(s)=Hg(l)��1/2O2(g) ��H����91 kJ��mol��1
D��H2O2(l)= 1/2O2(g)��H2O(l) ��H����98 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ���������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵�����ʾ������ȷ����( )
A�������������������������ֱ���ȫȼ�գ����߷ų�������
B���ɡ�C(ʯī)===C(���ʯ) ��H��1.9 kJ��mol����֪�����ʯ��ʯī�ȶ�
C����101 kPaʱ��2 g H2 ��ȫȼ������Һ̬ˮ���ų�285.8 kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)��O2(g)===2H2O(l) ��H����285.8 kJ/mol
D����ϡ��Һ�У�H����OH��===H2O ��H����57.3 kJ��mol ��������1 mol H2SO4�뺬2 mol NaOH����Һ��ϣ��ų�����������114.6 kJ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com