
 2NH3��g��
2NH3��g��| 716.8L |
| 22.4L/mol |
 ����2NH3��
����2NH3��| 8mol |
| 12mol+(b-12)mol+8mol |
| 4mol |
| 16mol |
| 12mol |
| 24mol |


 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��㶫ʡ����������������ѧ�������ϣ��¿���ѧ�Ծ��������棩 ���ͣ������
 2NH3��g��
2NH3��g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
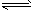 2NH3��g��
2NH3��g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���amolN2��bmolH2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
(1)����Ӧ���е�ijʱ��tʱn1(N2)=13mol,n1(NH3)=6moL,����a= ��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8L������£�������NH3���������Ϊ25%����ƽ��ʱNH3�����ʵ���Ϊ ��
(3)ԭ���������ƽ��������������ʵ���֮��Ϊ��д�����������,��ͬ�� ��
��4��ԭ��������У�a:b= ��
��5����ƽ��ʱ��N2��H2��ת����֮��Ϊ ��
��6��ƽ���������У�n(N2):n(H2):n(NH3)= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com