
| A�� | X2+��XO4-�Ļ�ԭ���� | |
| B�� | ��Һ�пɷ�����Z2+2A2+�T2A3++2Z- | |
| C�� | ������ǿ����˳��Ϊ��XO4-��B2��Z2��A3+ | |
| D�� | Z2�ڢ������������������������ |
���� ��16H++10Z-+2XO4-�T2X2++5Z2+8H2O�У�ZԪ�صĻ��ϼ����ߣ�XԪ�صĻ��ϼ۽��ͣ�
��2A2++B2�T2A3++2BR-�У�AԪ�صĻ��ϼ����ߣ�BԪ�صĻ��ϼ۽��ͣ�
��2B-+Z2�TB2+2Z-�У�ZԪ�صĻ��ϼ۽��ͣ�BԪ�صĻ��ϼ����ߣ����������ԭ��Ӧ��������������������Դ�����������������ԡ���ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�������
��� �⣺A����Ӧ��16H++10Z-+2XO4-=2X2++5Z2+8H2O�У�XԪ�ػ��ϼ۽��ͣ�����ԭ��X2+�ǻ�ԭ�����A��ȷ��
B����16H++10Z-+2XO4-�T2X2++5Z2+8H2O�������ԣ�XO4-��Z2��
��2A2++B2�T2A3++2B-�������ԣ�B2��A3+��
��2B-+Z2�TB2+2Z-�������ԣ�Z2��B2��
���������Թ�ϵΪ��XO4-��Z2��B2��A3+��������Һ�пɷ�����Z2+2A2+�T2A3++2Z-����B��ȷ��
C����B������֪�����Թ�ϵΪ��XO4-��Z2��B2��A3+����C����
D������Z�Ļ��ϼ����ߣ���Z2�ڢ����������������ZԪ�صĻ��ϼ۽��ͣ���Z2������������D��ȷ��
��ѡC��
���� ���⿼��������ԭ��Ӧ�������ԵıȽϼ���صĻ��������ȷ��Ӧ��Ԫ�صĻ��ϼ۱仯�������ԱȽϷ���Ϊ���Ĺؼ�����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����Ʒ��Na2O2�ĺ�����
����Ʒ��Na2O2�ĺ�����
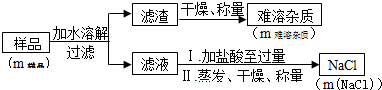
| m��Ʒ | m�������� | m��NaCl�� |
| 8.00g | 0.42g | 10.53g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �κ����嵥���ڱ�״�������ԼΪ22.4L������2NA��ԭ�� | |
| B�� | ���³�ѹ�£�16g������32 g������O3��������ԭ������Ϊ3NA | |
| C�� | ���³�ѹ�£�11.2L�����к��е���ԭ����Ϊ2NA | |
| D�� | ��״���£�0.3mol������̼�к�����ԭ����0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʵ���ɫ���� | B�� | ���ʵ��۷е����� | ||
| C�� | ���ʵ��ܶ���С | D�� | ������ˮ�е��ܽ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ���ᱣ������ɫ�����Լ�ƿ�� | |
| B�� | �������Ʊ�����ú���� | |
| C�� | ���Ƶ���ˮͨ����������ɫ�����Լ�ƿ�� | |
| D�� | ����������Һ�ô�ĥ�ڲ���������ͨ�Լ�ƿ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ���ڴ����У�CaCO3+2CH3COOH�TCa2++2CH3COO-+CO2��+H2O | |
| B�� | ������ͭ��Һ�����������壺Cu2++S2-�TCuS�� | |
| C�� | ϡ�����м���������ۣ�3Fe+8H++2NO3-�T3Fe3++2NO��+4H2O | |
| D�� | ��NaHCO3��Һ�м���������Ba��OH��2��Һ��Ba2++HCO3-+OH-�TBaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
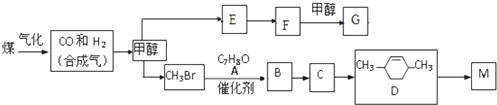
 +RX$\stackrel{����}{��}$
+RX$\stackrel{����}{��}$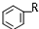 +HX���������գ�
+HX���������գ� 2HCHO+2H2O��
2HCHO+2H2O��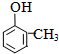 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com