
¢Ł½«5£„Na2CO3ČÜŅŗ¼ÓČėµ½Ź¢ÓŠ·ĻĢśŠ¼µÄÉÕ±ÖŠ(½žĆ»)£¬¼ÓČČŹż·ÖÖÓŗ󣬽«Na2CO3ČÜŅŗ³żČ„£¬Č»ŗóÓĆĖ®Ļ“µÓ2”Ŗ3±é”£
¢ŚĻņĻ“µÓ¹żµÄ·ĻĢśŠ¼ÖŠ¼ÓČė¹żĮæµÄĻ”H2SO4£¬æŲÖĘĪĀ¶ČŌŚ50”ę”Ŗ80”ęÖ®¼äÖĮĢśŠ¼ŗľ””£
¢Ū³ĆČČ¹żĀĖ£¬½«ĀĖŅŗ×ŖČėµ½ĆܱÕČŻĘ÷ÖŠ¾²ÖĆ£¬ĄäČ“½į¾§”£
¢Ü“ż½į¾§Ķź±ĻĀĖ³ö¾§Ģ壬ÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģå2”Ŗ3“Ī£¬ŌŁÓĆĀĖÖ½½«¾§ĢåĪüøÉ”£
¢Ż½«ÖĘµĆµÄ¾§Ģå·ÅŌŚŅ»øöŠ”¹ćæŚĘæÖŠ£¬ĆÜ·ā±£“ę”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃé¢ŁµÄÄæµÄŹĒ______________£¬ĘäÖŠ¼ÓČȵÄŌŅņŗĶ×÷ÓĆŹĒ_______________________
________________________________________________________________ӣ
(2)ŹµŃé¢ŚĆ÷ĻŌ²»ŗĻĄķ£¬ĄķÓÉŹĒ__________________________________________________”£
(3)ŹµŃé¢ÜÖŠÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģ壬ĘäÄæµÄŹĒ£ŗ______________£¬______________”£
 Ņ»ĻßĆūŹ¦ĢįÓÅŹŌ¾ķĻµĮŠ“š°ø
Ņ»ĻßĆūŹ¦ĢįÓÅŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÖ¾ŗčĻµĮŠČ«ÓÅÉč¼Ę±ŲŠŽŅ»»ÆѧĖÕ½Ģ°ę ĖÕ½Ģ°ę ĢāŠĶ£ŗ058
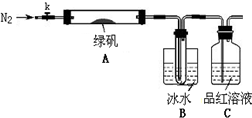
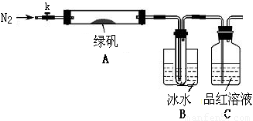
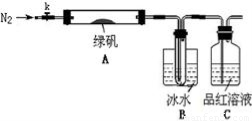
ijĶ¬Ń§ĶعżČēĻĀŹµŃéÓɹ¤Ņµ·ĻĢśŠ¼ÖʱøFeSO4”¤7H2O¾§Ģå£ŗ
¢Ł½«5£„””Na2NO3ČÜŅŗ¼ÓČėµ½Ź¢ÓŠ·ĻĢśŠ¼µÄÉÕ±ÖŠ(½žĆ»)£¬¼ÓČČŹż·ÖÖÓŗ󣬽«Na2CO3ČÜŅŗ³żČ„£¬Č»ŗóÓĆĖ®Ļ“µÓ2£3±é£®
¢ŚĻņĻ“µÓ¹żµÄ·ĻĢśŠ¼ÖŠ¼ÓČė¹żĮæµÄĻ”H2SO4£¬æŲÖĘĪĀ¶ČŌŚ50£80”ęÖ®¼äÖĮĢśŠ¼ŗľ”£®
¢Ū³ĆČČ¹żĀĖ£¬½«ĀĖŅŗ×ŖČėµ½ĆܱÕČŻĘ÷ÖŠ¾²ÖĆ£¬ĄäČ“½į¾§£®
¢Ü“ż½į¾§Ķź±ĻĀĖ³ö¾§Ģ壬ÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģå2£3“Ī£¬ŌŁÓĆĀĖÖ½½«¾§ĢåĪüøÉ£®
¢Ż½«ÖĘµĆµÄ¾§Ģå·ÅŌŚŅ»øöŠ”¹ćæŚĘæÖŠ£¬ĆÜ·ā±£“ę£®
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃé¢ŁµÄÄæµÄŹĒ________£¬ĘäÖŠ¼ÓČȵÄŌŅņŗĶ×÷ÓĆŹĒ________£®
(2)ŹµŃé¢ŚĆ÷ĻŌ²»ŗĻĄķ£¬ĄķÓÉŹĒ________£®
(3)ŹµŃé¢ÜÖŠÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģ壬ĘäÄæµÄŹĒ£ŗ________£¬________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢Ł½«5%Na2CO3ČÜŅŗ¼ÓČėµ½Ź¢ÓŠ·ĻĢśŠ¼µÄÉÕ±ÖŠ(½žĆ»)£¬¼ÓČČŹż·ÖÖÓŗ󣬽«Na2CO3ČÜŅŗ³żČ„£¬Č»ŗóÓĆĖ®Ļ“µÓ2”Ŗ3±é”£
¢ŚĻņĻ“µÓ¹żµÄ·ĻĢśŠ¼ÖŠ¼ÓČė¹żĮæµÄĻ”H2SO4£¬æŲÖĘĪĀ¶ČŌŚ50”Ŗ
¢Ū³ĆČČ¹żĀĖ£¬½«ĀĖŅŗ×ŖČėµ½ĆܱÕČŻĘ÷ÖŠ¾²ÖĆ£¬ĄäČ“½į¾§”£
¢Ü“ż½į¾§Ķź±ĻĀĖ³ö¾§Ģ壬ÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģå2”Ŗ3“Ī£¬ŌŁÓĆĀĖÖ½½«¾§ĢåĪüøÉ”£
¢Ż½«ÖĘµĆµÄ¾§Ģå·ÅŌŚŅ»øöŠ”¹ćæŚĘæÖŠ£¬ĆÜ·ā±£“ę”£
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃé¢ŁµÄÄæµÄŹĒ____________£¬ĘäÖŠ¼ÓČȵÄŌŅņŗĶ×÷ÓĆŹĒ________________________”£
(2)ŹµŃé¢ŚĆ÷ĻŌ²»ŗĻĄķ£¬ĄķÓÉŹĒ_________________________________________________”£
(3)ŹµŃé¢ÜÖŠÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģ壬ĘäÄæµÄŹĒ£ŗ____________£¬____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ½Ī÷Ź”°ĖŠ£øßČżĻĀѧʌĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ĀĢ·Æ£ØFeSO4?7H2O£©ŹĒÖĪĮĘȱĢśŠŌʶŃŖµÄĢŲŠ§Ņ©”£Ä³Ń§Š£µÄ»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§¶ŌĀĢ·Æ½ųŠŠĮĖČēĻĀµÄĢ½¾æ£ŗ
FeSO4?7H2OµÄÖʱø
øĆ»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ŌŚŹµŃéŹŅĶعżČēĻĀŹµŃéÓÉ·ĻĢśŠ¼£Øŗ¬ÉŁĮæŃõ»ÆĶ”¢Ńõ»ÆĢśµČŌÓÖŹ£©ÖʱøFeSO4”¤7H2O¾§Ģå£ŗ
¢Ł½«5%Na2CO3ČÜŅŗ¼ÓČėµ½Ź¢ÓŠŅ»¶ØĮæ·ĻĢśŠ¼µÄÉÕ±ÖŠ£¬¼ÓČČŹż·ÖÖÓ£¬ÓĆĒćĪö·Ø³żČ„
Na2CO3ČÜŅŗ£¬Č»ŗ󽫷ĻĢśŠ¼ÓĆĖ®Ļ“µÓ2”«3±é”£
¢ŚĻņĻ“µÓ¹żµÄ·ĻĢśŠ¼ÖŠ¼ÓČė¹żĮæµÄĻ”ĮņĖį£¬æŲÖĘĪĀ¶ČŌŚ50”«80”ęÖ®¼äÖĮĢśŠ¼ŗľ”£»
¢Ū³ĆČČ¹żĀĖ£¬½«ĀĖŅŗ×ŖČėµ½ĆܱÕČŻĘ÷ÖŠ£¬¾²ÖĆ”¢ĄäČ“½į¾§£»
¢Ü“ż½į¾§Ķź±Ļŗó£¬ĀĖ³ö¾§Ģ壬ÓĆÉŁĮæ±łĖ®Ļ“µÓ2”«3“Ī£¬ŌŁÓĆĀĖÖ½½«¾§ĢåĪüøÉ£»
¢Ż½«ÖʵƵÄFeSO4”¤7H2O¾§Ģå·ÅŌŚŅ»øöŠ”¹ćæŚĘæÖŠ£¬Ćܱձ£“ę”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃé²½Öč¢ŁµÄÄæµÄŹĒ”””””””””””””””””””””””””””£
£Ø2£©ŹµŃé²½Öč¢ŚĆ÷ĻŌ²»ŗĻĄķ£¬ĄķÓÉŹĒ”””””””””””””””””””””””””£
£Ø3£©ĪŖĮĖĻ“µÓ³żČ„¾§Ģå±ķĆęø½×ŵÄĮņĖįµČŌÓÖŹ£¬ŹµŃé²½Öč¢ÜÖŠÓĆÉŁĮæ±łĖ®Ļ“µÓ¾§Ģ壬ŌŅņŹĒ”””””””””””””””””””””””””””””””””””””” ”£
£Ø¶ž£©Ģ½¾æĀĢ·Æ£ØFeSO4”¤7H2O£©ČČ·Ö½āµÄ²śĪļ
 ŅŃÖŖSO3µÄČŪµćŹĒ16.8”ćC£¬·ŠµćŹĒ44.8”ćC£¬øĆŠ”×éÉč¼ĘČēĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£ØĶ¼ÖŠ¼ÓČČ”¢¼Š³ÖŅĒĘ÷µČ¾łŹ”ĀŌ£©£ŗ
ŅŃÖŖSO3µÄČŪµćŹĒ16.8”ćC£¬·ŠµćŹĒ44.8”ćC£¬øĆŠ”×éÉč¼ĘČēĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£ØĶ¼ÖŠ¼ÓČČ”¢¼Š³ÖŅĒĘ÷µČ¾łŹ”ĀŌ£©£ŗ
”¾ŹµŃé¹ż³Ģ”æ
¢ŁŅĒĘ÷Į¬½Óŗ󣬼ģ²é×°ÖĆAÓėBĘųĆÜŠŌ£»
¢ŚČ”Ņ»¶ØĮæĀĢ·Æ¹ĢĢåÖĆÓŚAÖŠ£¬ĶØČėN2ŅŌĒż¾”×°ÖĆÄŚµÄæÕĘų£¬¹Ų±Õk£¬ÓĆ¾Ę¾«µĘ¼ÓČČÓ²ÖŹ²£Į§¹Ü£»
¢Ū¹Ū²ģµ½A ÖŠ¹ĢĢåÖš½„±äŗģ×ŲÉ«£¬BÖŠŹŌ¹ÜŹÕ¼Æµ½ĪŽÉ«ŅŗĢ壬CÖŠČÜŅŗĶŹÉ«£»
¢Ü“żAÖŠ·“Ó¦ĶźČ«²¢ĄäČ“ÖĮŹŅĪĀŗó£¬Č”ÉŁĮæ·“Ó¦ŗó¹ĢĢåÓŚŹŌ¹ÜÖŠ£¬¼ÓČėĮņĖįČܽā£¬Č”ÉŁĮæµĪČė¼øµĪKSCNČÜŅŗ£¬ČÜŅŗ±äŗģÉ«£»
¢ŻĶłB×°ÖƵďŌ¹ÜÖŠµĪČė¼øµĪBaCl2ČÜŅŗ£¬ČÜŅŗ±ä»ė×Ē”£
(4£©ŹµŃé½į¹ū·ÖĪö
½įĀŪ1£ŗBÖŠŹÕ¼Æµ½µÄŅŗĢåŹĒ?????????????????? £»
½įĀŪ2£ŗCÖŠČÜŅŗĶŹÉ«£¬æÉĶĘÖŖ²śĪļÖŠÓŠ???? ?????????????? £»
½įĀŪ3£ŗ×ŪŗĻ·ÖĪöÉĻŹöŹµŃé¢ŪŗĶ¢ÜæÉĶĘÖŖ¹ĢĢå²śĪļŅ»¶ØÓŠFe2O3”£
”¾ŹµŃé·“Ė¼”æ
£Ø5£©ĒėÖø³öøĆŠ”×éÉč¼ĘµÄŹµŃé×°ÖƵÄĆ÷ĻŌ²»×ć£ŗ??????????????????????????? ”£
£Ø6£©·Ö½āŗóµÄ¹ĢĢåÖŠæÉÄÜŗ¬ÓŠÉŁĮæFeO£¬Č”ÉĻŹöŹµŃé¢ÜÖŠŃĪĖįČܽāŗóµÄČÜŅŗÉŁŠķÓŚŹŌ¹ÜÖŠ£¬Ń”ÓĆŅ»ÖÖŹŌ¼Į¼ų±š£¬øĆŹŌ¼Į×īŗĻŹŹµÄŹĒ?????????? ”£
a£®ĀČĖ®ŗĶKSCNČÜŅŗ???? b£®ĖįŠŌKMnO4ČÜŅŗ????? c£®H2O2???? d£®NaOHČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com