
ij�¶�(t ��)ʱ��ˮ�����ӻ�ΪKW��1.0��10��13mol2��L��2������¶�(����ڡ�����С�ڡ����ڡ�)________25 �棬��������_______________________________________��
�������¶���pH��11�Ŀ�������Һa L��pH��1��ϡ����b L���(���Ϻ���Һ�����С�仯���Բ���)����ͨ��������д���²�ͬ���ʱ������Һ������ȣ�
(1)�����û��ҺΪ���ԣ���a��b��________������Һ�и������ӵ�Ũ���ɴ�С����˳����___________________________________��
(2)�����û��Һ��pH��2����a��b��________������Һ�и������ӵ�Ũ���ɴ�С����˳����__________________________________________��
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʵ��ȷ��ij��HA��������ʡ��ס�����ͬѧ�ķ����ǣ�
�ף��ٳ�ȡһ��������HA����0.1 mol/L��HA��Һ100 mL������pH��ֽ�������Һ��pH������֤��HA��������ʡ�
�ң�������֪���ʵ���Ũ�ȵ�HA��Һ�����ᣬ�ֱ�����pH��1����������Һ��100 mL���ڷֱ�ȡ��������Һ��10 mL����ˮϡ����100 ml���۸�ȡ��ͬ���������ϡ��Һװ�������Թ��У�ͬʱ���봿����ͬ��п�����۲�������֤��HA��������ʡ�
(1)�����У�˵��HA��������ʵ������Dz����Һ��pH________1(�>����<������)���ҷ����У�˵��HA��������ʵ�������________(�����)��
a��װ������Թ��зų�H2�����ʿ�
b��װHA��Һ���Թ��зų�H2�����ʿ�
c�������Թ��в������������һ����
(2)���������ҷ���������ʵ��֮���Ͳ���֮��________��
(3)���������һ���������Ƚ������еķ���(ҩƷ������)�������ʵ�鷽����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl����SO42�������ʣ��ᴿ�����������£�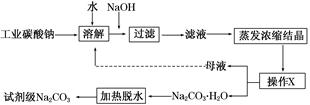
��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��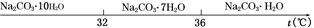
��.�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ��,�������ʵ��:�����½�pH=2����������ҺHA��HB��pH=12��MOH����Һ��1 mL,�ֱ��ˮϡ�͵�1 000 mL,��pH�ı仯����Һ����Ĺ�ϵ��ͼ,��������������,��ش���������:
(1)HAΪ ��,HBΪ ��(�ǿ��������)��
(2)��c=9,��ϡ�ͺ��������Һ��,��ˮ�����������Ũ�ȵĴ�С˳��Ϊ (���ᡢ�ѧʽ��ʾ)��
(3)��c=9,��ϡ�ͺ��HA��Һ��MOH��Һȡ��������,��������Һ��c(A-)��c(M+)�Ĵ�С��ϵΪc(A-) (����ڡ�����С�ڡ����ڡ�)c(M+)��
(4)��b+c=14,��MOHΪ ��(�ǿ��������)����ϡ�ͺ��HB��Һ��MOH��Һȡ��������,���û����Һ��pH 7(����ڡ�����С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�ֱ�ΪNH4HSO4��Ba(OH)2��AlCl3��Na2CO3 4�������е�1�֣�����ˮ����ȫ���룬�ֽ�������ʵ�飺
������A��Һ��B��Һ��Ϲ��ȿ����ɳ����ʹ̼�����ζ���壻
������A��Һ��C��Һ��Ͽ����ɳ����ң�
��A��Һ��B��Һ�����ܽ�����ң����������ܽ�����ס�
��ش�
(1)A�Ļ�ѧʽΪ________������ʱ����pH��ȵ�A��Һ��D��Һ�ֱ�ϡ��10����pH�ֱ��Ϊa��b����a________b(�>����������<��)��
(2)��������C��Һ�����գ�������ù���Ϊ________(�ѧʽ)��
(3)C��Һ��D��Һ��Ӧ�����ӷ���ʽΪ__________________________________________
(4)��B��Һ����μ��������������ʵ���Ũ�ȵ�NaOH��Һ���μӹ�����ˮ�ĵ���ƽ�⽫________(���������������)�ƶ�������������Һ�и�����Ũ���ɴ�С��˳��Ϊ________________________________________________________________________��
(5)��֪������Ksp��x����0.03 mol��L��1��A��Һ��0.01 mol��L��1��B��Һ�������ϣ������Һ��������ӵ�Ũ��Ϊ________(�ú�x�Ĵ���ʽ��ʾ����Ϻ���Һ����仯���Բ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʵĵ���ƽ�⡢�����ˮ��ƽ�����������ܽ�ƽ������ڻ�ѧƽ�⡣
��֪H2A��ˮ�д�������ƽ�⣺H2A=H����HA����HA�� H����A2����
H����A2����
(1)������NaHA��Һ��pH________(����ţ���ͬ)��ԭ����_____________��
A������7������������ B��С��7 C������7 D����ȷ��
(2)ij�¶��£�����0.1 mol��L��1��NaHA��Һ����εμ�0.1 mol��L��1 KOH��Һ����Һ������(���Ի�Ϻ���Һ������仯)����ʱ�û����Һ�е����й�ϵһ����ȷ����________��
A��c(H��)��c(OH��)��1.0��10��14
B��c(Na��)��c(K��)��c(HA��)��2c(A2��)
C��c(Na��)>c(K��)
D��c(Na��)��c(K��)��0.05 mol��L��1
(3)��֪������H2A�ĸ���(CaA)�ı�����Һ�д�������ƽ�⣺CaA(s)??Ca2��(aq)��A2��(aq)����H>0����Ҫʹ����Һ��Ca2��Ũ�ȱ�С���ɲ�ȡ�Ĵ�ʩ��________��
A�������¶ȡ����������� B�������¶�
C������NH4Cl���� D������Na2A����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Ԫ��(��ѧʽ��H2B��ʾ)��ˮ�еĵ��뷽��ʽ��H2B===H����HB����HB�� H����B2�����ش��������⡣
H����B2�����ش��������⡣
(1)Na2B��Һ��_____(����ԡ��������ԡ����ԡ�)��������_______(�����ӷ���ʽ��ʾ)��
(2)��0.1 mol��L��1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����____��
A��c(B2��)��c(HB��)��c(H2B)��0.1 mol��L��1
B��c(Na��)��c(OH��)��c(H��)��c(HB��)
C��c(Na��)��c(H��)��c(OH��)��c(HB��)��2c(B2��)
D��c(Na��)��2c(B2��)��2c(HB��)
(3)��֪0.1 mol��L��1 NaHB��Һ��pH��2����0.1 mol��L��1 H2B��Һ�е������ӵ����ʵ���Ũ�ȿ���____0.11 mol��L��1(�<������>������)��������_____��
(4)0.1 mol��L��1 NaHB��Һ�и�������Ũ���ɴ�С��˳����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���ijһԪ��HA(�ס��ҡ�������������ͬ��һԪ��)��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH���±���ʾ��
| ʵ�� ��� | HA���ʵ��� Ũ��/(mol��L��1) | NaOH���ʵ��� Ũ��/(mol��L��1) | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH��a |
| �� | 0.12 | 0.1 | pH��7 |
| �� | 0.2 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH��10 |
 H����B2��
H����B2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ij��Һ��ֻ����OH����H+��NH4+��Cl���������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
| A��c(NH4+)��c(Cl��)��c(OH��)��c(H+) | B��c(Cl��)��c(H+)��c(NH4+)��c(OH��) |
| C��c(Cl��)��c(NH4+)��c(H+)��c(OH��) | D��c(NH4+)��c(OH��)��c(Cl��)��c(H+) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com