
����Ŀ����HCl����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3��������1L 1mol/L��ϡ���ᡣ�ش������й����⡣��������������裺
��1�����㣺����ȡ36.5%��Ũ��������Ϊ________������
��2����ȡ������Ͳ��ȡ����Ũ���Ტע�뵽250mL�ձ��У�
��3���ܽ⣺___________________________________________________����ȴ��
��4��ת�ƣ���Һ��:_________________________________________________��
��5��ϴ�ӣ�__________________________________________________________��
��6�����ݣ�__________________________________________________________��
��7��ҡ�ȣ��Ǻ�����ƿ���������ߵ���ҡ�ȣ�
��8�����أ������úõ�ϡ���ᵹ���Լ�ƿ�У������ñ�ǩ����ǩ��Ҫע��______________��
��9�������������²��������õ�����Ũ���ǡ�ƫ�ߡ�������ȡ����ǡ�ƫ�͡���
������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�____________��
������ƿ��������������ˮ��__________________��
��û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У�___________��
�ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�________________��
�ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��________________��
��δ��ȴ��ת�ƶ��ݣ�_____________________��
����ϴ������ƿ��_______________��
���𰸡� 83.3 ��ʢ��Ũ������ձ��м���Լ100mLˮ���ò������������裬ʹ���Ͼ��� ���ձ��е���Һ�ز�����ת�Ƶ�����ƿ�� ����������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��ҺҲת�Ƶ�����ƿ��.Ȼ��ҡ�� ������ƿ�м�ˮ����Һ����̶�1--2cm����Һ��ӽ��̶��ߣ�ʱ���ý�ͷ�ι���εμ�ˮ����Һ����ʹ���̶������� ��ǩ��Ҫע��ϡ���ᡢ1mol/L ƫ�� ��� ƫ�� ƫ�� ƫ�� ƫ�� ƫ��
����������1����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3�����ʵ���Ũ��C=![]() =12mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�V��12mol/L=1mol/L��1000mL�����V=83.3mL��
=12mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�V��12mol/L=1mol/L��1000mL�����V=83.3mL��
(3). ��ʢ��Ũ������ձ��м���Լ100mLˮ���ò������������裬ʹ���Ͼ��� (4). ���ձ��е���Һ�ز�����ת�Ƶ�����ƿ�� (5). ����������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��ҺҲת�Ƶ�����ƿ��.Ȼ��ҡ�� (6). ������ƿ�м�ˮ����Һ����̶�1--2cm����Һ��ӽ��̶��ߣ�ʱ���ý�ͷ�ι���εμ�ˮ����Һ����ʹ���̶������� (8). ��ǩ��Ҫע��ϡ���ᡢ1mol/L ��9����������������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�������ȡŨ�������ƫ������ƫ�࣬��ҺŨ��ƫ�ߣ�������ƿ��������������ˮ�������ʵ����ʵ�������Һ���������Ӱ�죬��ҺŨ����ȣ���û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У����²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ��ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��������Һ���ƫ����ҺŨ��ƫ�ͣ���δ��ȴ��ת�ƶ��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�����ϴ������ƿ�������������ʵ���ƫ����ҺŨ��ƫ�ߣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������ɳ�����չ������ء����б仯�����ڻ�ѧ�о��������( )
A. MERS������������� B. ���ʵ�ԭ�ӵ���ըʵ��
C. ���ڳ���������ԭ��̽�� D. ����ɽ������ұ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧһѡ��3�����ʽṹ�����ʡ�
±��Ԫ�ص������������ʷ�Ӧ�γɶ��ֻ������������ѧ���ʽṹ�����ʵ����֪ʶ�ش�
(1)д����̬��ԭ�ӵļ۵����Ų�ʽ___________��
(2)±��Ԫ�صĺ�������������ǿ����____(д��ѧʽ)����������ӵ����幹��Ϊ_________��
(3)�Ƚ��������±������۵�ͷе㣬������仯���ɼ�ԭ��_________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | -49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
(4)��֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6(![]() )��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
)��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
(5)��֪��Ԫ����������������ֻ��1�����ӡ�����������ԭ�ӹ�����������ӵ�Ԫ��M�γɵ�һ�ֻ����������������ͼ��ʾ��
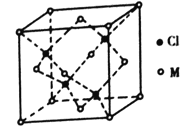
�ٸû�����Ļ�ѧʽΪ_______����֪��������a=0.542 nm���˾�����ܶ�Ϊ_____g/cm3��(д������ʽ����Ҫ��������������ӵ�����ΪNA)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʹ�õ�������1L 0.1mol/L������ͭ��Һ����ȷ�������ǣ�
A. ���������ȳ�ȥ�ᾧˮ��,��ȡ16 g����1Lˮ��
B. ��ȡ����25 g����1Lˮ��
C. ��25 g������������ˮ,Ȼ����Һϡ����1L
D. ��16 g��������ˮ��Ȼ����Һϡ����1L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼Ԫ�ؼ��仯������������������ϢϢ��أ���ش��������⣺
��1����Ȼ���е�̼ѭ��������������뷢չ������Ҫ���塣
����ɫֲ��Ĺ����������CO2�ͷ�O2�Ĺ��̿�������Ϊ����������
2CO2(g)+2H2O(l)+2C5H10O4(s) = 4(C3H6O3)+(s)+O2(g)+4e- ��H=+1360 kJ��mol-1
12(C3H6O3)+(s)+12e- = C6H12O6(s��������)+6C5H10O4(s)+3O2(g) ��H=-1200 kJ��mol-1
����ɫֲ�����ö�����̼��ˮ�ϳ������Dz��ų��������Ȼ�ѧ����ʽΪ��_________________��
���ܶ����γ���ʯ�����е���Ҫ�ɷ�̼�����һ���������ܽ�ͳ����γɣ���վ�ڻ�ܵĽǶȽ����ܶ��γɹ��̼�Ϊ������ԭ��____________��
(2) ��ҵ��̼���仯��������Ҫ�Ĺ�ҵԭ�ϡ�
����CO2��NaCl��NH3Ϊԭ���Ƶ�Na2CO3���������Ƽ������Ҫ���裬������ʵ��ܽ��������ͼ��ʾ����д�����������Ȼ�����Һ���Ⱥ�ͨ��������NH3��CO2������Ӧ�����ӷ���ʽ��___��

���³�ѹ�£���NaHCO3��Һ�д�������ƽ�⣺
HCO3-+H2O![]() H2CO3+OH- Kh=2.27��10-8
H2CO3+OH- Kh=2.27��10-8
HCO3- ![]() CO32-+H+ Ka2=4.7��10-11
CO32-+H+ Ka2=4.7��10-11
H2O![]() H++OH- Kw=1.0��10-14
H++OH- Kw=1.0��10-14
����K��������NaHCO3��Һ�Լ��Ե�ԭ��____________����NaHCO3��Һ�м���ͨ��CO2������Һ��n(HCO3-)��n(H2CO3)= ____________ʱ��Һ���Դ����ԡ�
�ڹ�ҵ�Ͽ������ü״��Ʊ�������
�״������������Ʊ������ĺ���Ң��ӦΪCH3OH(g) ![]() CO(g)+2H2(g),�����ݻ�Ϊ2.0L���ܱ������г���0.60mol��CH3OH(g)��ϵѹǿΪp1����һ�������´ﵽƽ��ʱ����ϵѹǿΪp2����p2��p1=2.2�����������CH3OH��ƽ��ת����Ϊ__________________��
CO(g)+2H2(g),�����ݻ�Ϊ2.0L���ܱ������г���0.60mol��CH3OH(g)��ϵѹǿΪp1����һ�������´ﵽƽ��ʱ����ϵѹǿΪp2����p2��p1=2.2�����������CH3OH��ƽ��ת����Ϊ__________________��
��ҵ�ϻ��������ü״������������Ʊ���������һ���¶�����Ag/CeO2-ZnOΪ������ԭ���������Է�Ӧѡ����(ѡ����Խ��ʾ���ɵĸ�����Խ��)Ӱ���ϵ��ͼ��ʾ.��
n(O2)/n(CH3OH)=0.25ʱ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ__________�����Ʊ�H2ʱ��ÿ���n(O2)/n(CH3OH)=________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���������ơ���Ͷ��һ������ϡ�����У�����������ʱ��仯����������ͼ��ʾ��������˵������ȷ����
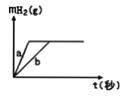
A. Ͷ���Na��Kһ��������
B. Ͷ���Na����������K������
C. ����aΪK��bΪNa
D. ϡ�������һ���Dz�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���ֻ������������ͬ��һ��Ԫ��X��C��D��ˮ��Һ�ֱ���AgNO3��Һ���ʱ�����е�Ԫ��X��Ag+��ϳɲ�����ϡHNO3�İ�ɫ������A��B��E�е�XԪ�����ܡ�A�ڴ����ͼ��������¿ɷֽ�����C������F��F��ʹ�������ľ����ȼ��D��KOH��Һ��Ӧ����C��ˮ��B��A�����Ԫ����ȫ��ͬ����B��ˮ��Һ��ͨ��CO2����E������һ�����ʣ�E����ֽ�����D��F���Իش�
��1��д��A��B��C��D��E��F�������ʵĻ�ѧʽ��
A________��B________��C_______��D________��E________��F________��
��2��д������ָ����Ӧ�Ļ�ѧ����ʽ��
A�ֽ�����C��F��___________________________________��
E����ֽ⣺________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з���ʽ��ʾ������һ���Ǵ��������( )
A��C5H10 B ��C8H10 C ��CH4O D �� C2H4Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�M���˵����ȷ����(����)
A. ��ʾ�ڶ����Ӳ�B. ���������8������
C. ��ʾ�������Ӳ�D. ����Ӧ�����2������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com