
 ��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�
��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�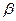 ��﮻�ʯΪԭ���Ʊ�̼��﮵�һ���������£�
��﮻�ʯΪԭ���Ʊ�̼��﮵�һ���������£�
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 2.7 | 3.7 | 9.6 |
| ��ȫ����pH | 3.7 | 4.7 | 11 |
| �¶�/�� | 0 | 10 | 20 | 50 | 75 | 100 |
| Li2CO3���ܽ��/g | 1.539 | 1.406 | 1.329 | 1.181 | 0.866 | 0.728 |
| A�������� | B�������� | C������̨������Ȧ�� | D���ƾ��� E��Բ����ƿ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
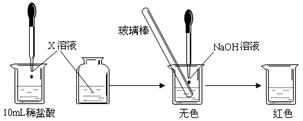
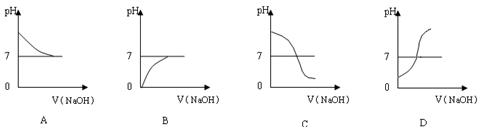
| �ζ����� | ��������/mL | NaOH��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 2.00 | 20.20 |
| 2 | 25.00 | 1.02 | 21.03 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | ʵ�鲽�� |
| �� | ��þ����ɰֽ��ĥ�����ˮ�У�������Һ�еμӷ�̪��Һ |
| �� | �����Ƶõ���Na2S��Һ�еμ����Ƶ���ˮ |
| �� | ��һС������Ʒ�����з�̪��Һ����ˮ�� |
| �� | ��þ��Ͷ��ϡ������ |
| �� | ������Ͷ��ϡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
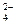 ��CO
��CO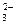 ��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�
��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
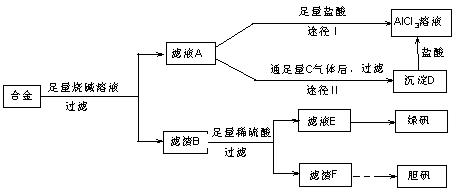
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
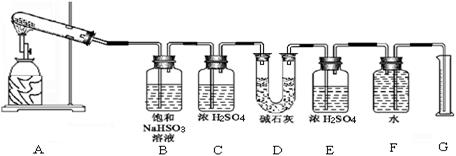
 �������ϴ����������� ��
�������ϴ����������� ��| ʵ��С�� | ��ȡCaSO4 ������(g) | װ��D���� ������(g) | ��ȡ���������װ�ò������������ (����ɱ�״������������) (mL) |
| һ | 4.08 | 2. 56 56 | 224 |
| �� | 5.44 | 2.56 | 448 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�| A��BaCl2 | B��NaOH | C��Na2SO4 | D��HCl |
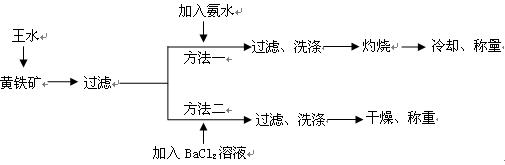
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
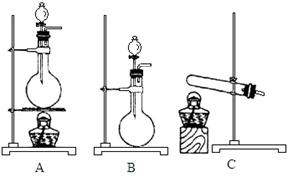
 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������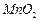 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ�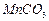 �IJ����������£�
�IJ����������£�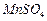 ��Һ��
��Һ��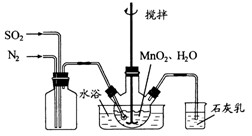
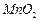 ��ˮ�����裬ͨ��
��ˮ�����裬ͨ��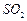 ��
��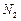 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��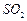 ��������ӦƬ�̣����ˣ���֪
��������ӦƬ�̣����ˣ���֪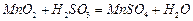 ����
����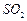 ������ת����ȫ����ͨ��
������ת����ȫ����ͨ��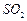 ��
��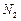 ����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��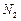 ���ɿ�������÷�ӦҺ��
���ɿ�������÷�ӦҺ��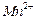 ��
��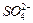 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��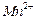 ��
��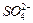 Ũ�ȱ仯�������Բ����ԭ���� ��
Ũ�ȱ仯�������Բ����ԭ���� ��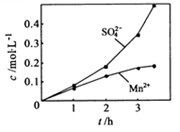
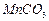 ���壺��֪
���壺��֪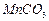 ������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻
������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻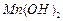 ��ʼ����ʱ
��ʼ����ʱ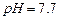 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�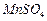 ��Һ�Ʊ��ߴ�
��Һ�Ʊ��ߴ�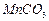 �IJ�������[ʵ���п�ѡ�õ��Լ���
�IJ�������[ʵ���п�ѡ�õ��Լ���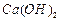 ��
��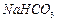 ��
��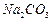 ��
��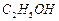 ]��
]���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ij��Һ���Ƿ��м�ȩ����ʢ��2mL10������ͭ��Һ���Թ��У��μ�4~8��10��������������Һ����Ͼ��ȣ��������Һ������ |
| B��ʵ��������ϩ����Ũ��������ˮ�Ҵ��������3��1��Ϻ�Ѹ�ټ�����170�� |
| C����ȡ�屽������м��Һ�塢����ֻ�Ϻ���� |
| D����ȡ�ܽ���ˮ�е������⣺����ֲ���������á��ֲ��ȡ���л����ٷ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com