
ÎŞ˛â¶¨ÁňËáŃÇĚú茶§Ě塾Ł¨NH4Ł©2Fe (SO4)2 ˇ¤ xH2OˇżÖĐĚúµÄş¬ÁżŁ¬ÄłĘµŃéС×é×öÁËČçĎÂʵŃ飺
˛˝ÖčŇ»ŁşÓõç×ÓĚěƽ׼ȷłĆÁż5.000gÁňËáŃÇĚú茶§Ě壬ĹäÖĆłÉ250mlČÜŇşˇŁ
˛˝Öč¶ţŁşČˇËůĹäČÜŇş25.00mlÓÚ׶ĐÎĆżÖĐŁ¬ĽÓϡH2SO4ËữŁ¬ÓĂ0.010mol/L KMnO4ČÜŇşµÎ¶¨ÖÁFe2+ǡşĂČ«˛żŃő»ŻłÉFe3+Ł¬Í¬Ę±Ł¬MnO4-±»»ąÔłÉMn2+ˇŁ
ÔŮÖظ´˛˝Öč¶ţÁ˝´ÎˇŁ
Çë»Ř´đĎÂÁĐÎĘĚ⣺
Ł¨1Ł©ĹäÖĆÁňËáŃÇĚúď§ČÜŇşµÄ˛Ů×÷˛˝ÖčŇŔ´ÎĘÇŁşłĆÁżˇ˘ ˇ˘×ŞŇơ˘Ď´µÓ˛˘×ŞŇơ˘ ˇ˘ŇˇÔȡŁ
Ł¨2Ł©ÓĂ µÎ¶¨ąÜʢ·ĹKMnO4ČÜŇşˇŁ
Ł¨3Ł©µ±µÎČë×îşóŇ»µÎKMnO4ČÜŇş,łöĎÖ ,Ľ´µ˝´ďµÎ¶¨Öյ㡣·´Ó¦µÄŔë×Ó·˝łĚĘ˝Łş
Ł¨4Ł©µÎ¶¨˝áąűČçϱíËůĘľŁş
| µÎ¶¨´ÎĘý | ´ý˛âČÜŇşµÄĚĺ»ý/mL | ±ę׼ČÜŇşµÄĚĺ»ý | |
| µÎ¶¨Ç°żĚ¶Č/mL | µÎ¶¨şóżĚ¶Č/mL | ||
| 1 | 25.00 | 1.05 | 21.04 |
| 2 | 25.00 | 1.50 | 24.50 |
| 3 | 25.00 | 0.20 | 20.21 |
Ł¨1Ł©Čܽ⡢¶¨ČÝ
Ł¨2Ł©ËáĘ˝
Ł¨3Ł©ČÜŇşÓÉ»ĆÉ«±äłÉ×ĎşěÉ«Ł¬ÇŇ°ë·ÖÖÓÄÚ˛»ÍĘÉ«Ł»
MnO4-+5Fe2++8 H+=Mn2++5Fe3++4H2O
Ł¨4Ł©11.20%
Ł¨5Ł©˘ŮĆ«¸ß ˘ÚÎŢÓ°Ďě ˘ŰB ˘ÜĆ«µÍ
˝âÎöĘÔĚâ·ÖÎöŁş
Ł¨1Ł©ĚúÄÜą»˝«ČýĽŰĚúŔë×Ó»ąÔłÉŃÇĚúŔë×ÓŁ¬ËůŇÔĚúąýÁżżÉŇÔ±ŁÖ¤ÁňËáŃÇĚúÖв»ş¬ÁňËáĚúŁ¬
Ł¨2Ł©ÓÉÓÚÔÚ0ˇ«60ˇć·¶Î§ÄÚŁ¬Í¬Ň»Î¶ČĎÂÁňËáŃÇĚú茶§ĚĺµÄČÜ˝â¶Č×îСŁ¬ËůŇÔÁňËáŃÇĚúşÍÁňËá淋ĻěşĎČÜŇşÖпɻńµĂÁňËáŃÇĚú茶§Ě壬
Ł¨3Ł©ľąýŐô·˘Ĺ¨ËőŁ¬ŔäČ´˝áľ§Ł¬ąýÂˡ˘±ůˮϴµÓŁ¬¸ÉÔď˛Ů×÷Ł¬żÉŇԵõ˝ÁňËáŃÇĚú茶§Ě壬
Ł¨4Ł©˘ŮĹäÖĆ100mLČÜŇşĐčŇŞµÄ˛ŁÁ§ŇÇĆ÷ÓĐŁş˛ŁÁ§°ôˇ˘100mLČÝÁżĆżˇ˘˝şÍ·µÎąÜˇ˘ÉŐ±Ł¬
˘ÚĐčŇŞÖŞµŔµÎ¶¨ĎűşÄµÄ¸ßĂĚËáĽŘČÜŇşµÄĚĺ»ýŁ¬Č»şó¸ůľÝ·´Ó¦ĽĆËăłöŃÇĚúŔë×ÓµÄÎďÖʵÄÁżŁ¬
żĽµăŁşÖƱ¸ĘµŃé·˝°¸µÄÉčĽĆ


 ĚěĚěĎňÉĎŇ»±ľşĂľíϵÁĐ´đ°¸
ĚěĚěĎňÉĎŇ»±ľşĂľíϵÁĐ´đ°¸ СѧÉú10·ÖÖÓÓ¦ÓĂĚâϵÁĐ´đ°¸
СѧÉú10·ÖÖÓÓ¦ÓĂĚâϵÁĐ´đ°¸
| Ä꼶 | ¸ßÖĐżÎłĚ | Ä꼶 | łőÖĐżÎłĚ |
| ¸ßŇ» | ¸ßŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ | łőŇ» | łőŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ß¶ţ | ¸ß¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ | łő¶ţ | łő¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ßČý | ¸ßČýĂâ·ŃżÎłĚÍĆĽöŁˇ | łőČý | łőČýĂâ·ŃżÎłĚÍĆĽöŁˇ |
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
(11·Ö)Äł»ŻŃ§Đˇ×éÉčĽĆʵŃéĚ˝ľżĚĽËáÄơ˘ĚĽËáÇâÄƵÄĐÔÖĘŁ¬ĘµŃéČçĎÂŁşČˇÁ˝Ö§ĘԹֱܷđĽÓČë10 mLĎŕͬŨ¶ČµÄϡŃÎËᣬ˝«Á˝¸ö¸÷×°ÓĐ0 5 gµÄNa2CO3şÍNaHCO3·ŰÄ©µÄСĆřÇň·Ö±đĚ×ÔÚÁ˝¸öĘÔąÜÉĎŁ¬˝«ĆřÇňÄڵĹĚĚĺ·ŰĩͬʱµąČËĘÔąÜÖĐŁ¬ŇŃÖŞŃÎËá×ăÁżŁ¬ąŰ˛ěʵŃéĎÖĎóˇŁ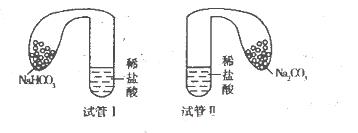
(l)Á˝Ö§ĘÔąÜÖĐľů˛úÉúĆřĚ壬ĆäÖвúÉúĆřĚĺ˝ĎżěµÄÎŞ________(ĚĘÔąÜIˇ±»ňˇ°ĘÔąÜIIˇ±)Ł¬Í¶Čë________Ł¨ĚѧʽŁ©µÄĘÔąÜÖĐĆřÇň±äµĂ±Č˝Ď´óˇŁ
Ł¨2Ł©Ľ×ͬѧ´ĄĂţÉĎĘöÁ˝ĘԹܣ¬·˘ĎÖĘÔąÜI±äŔ䣬ĘԹܢň±äČČŁ¬ÓÉ´ËĚáłö˛»ąÜĆä״̬ČçşÎŁ¬NaHCO3ÓëHCl·´Ó¦ÎŞÎüČČ·´Ó¦Ł¬Na2CO3ÓëHCl·´Ó¦ÎŞ·ĹČČ·´Ó¦ˇŁ
ÎŞ˝řŇ»˛˝Ě˝ľżNa2CO3ˇ˘NaHCO3ÓëŃÎËá·´Ó¦µÄÄÜÁż±ä»ŻŁ¬ŇŇͬѧ˝řĐĐĎÂÁĐʵŃ飬˛Ů×÷˛˝ÖčÎŞŁş˘ŮĎňĘÔĽÁ1ÖĐĽÓČëĘÔĽÁ2Ł¬˝Á°čˇ˘˛â¶¨Î¶ȣ»˘Úľ˛Öᢲⶨζȣ»˘ŰÔŮĽÓČëĘÔĽÁ3Ł®˝Á°čˇ˘˛â¶¨Î¶ȡ˘ĽÇÂĽˇŁµĂµ˝ŇÔĎÂĘýľÝŁş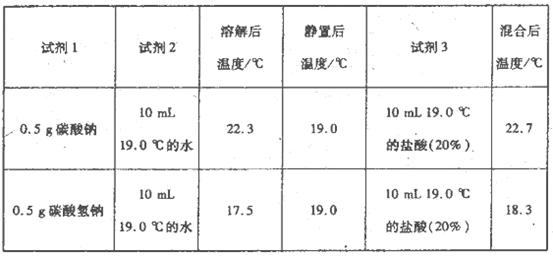
ÉĎĘöʵŃéĐčŇŞÓõ˝µÄ˛ŁÁ§ŇÇĆ÷ÓĐ________ˇŁ
ŇŇͬѧżÉµĂłö˝áÂŰŁş
˘ŮNaHCO3µÄČÜ˝âąýłĚ________Ł¨ĚÎüČȡ±»ňˇ°·ĹČȡ±Ł¬ĎÂͬŁ©Ł»Na2CO3µÄČÜ˝âąýłĚ________ˇŁ
˘ÚCO32ŁÓëHŁ«·´Ó¦ÎŞŇ»·´Ó¦Ł¨Ě·ĹČȡ±»ňˇ°ÎüČȡ±Ł¬ĎÂͬŁ©Ł¬HCO3ŁÓëHŁ«·´Ó¦ÎŞ________·´Ó¦ˇŁ
Ł¨3Ł©±Č˝ĎĽ×ŇŇͬѧµÄʵŃ飬ÄăČĎÎŞ ________Ł¨ĚĽ×ˇ±»ňˇ°Ňҡ±Ł©µÄʵŃé·˝°¸¸üşĎŔíˇ˘¸üŃĎĂܡŁ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
˛ÝËáĘÇŇ»ÖÖÖŘŇŞµÄ»Żą¤ÔÁĎŁ¬ąă·şÓĂÓÚŇ©ÎďÉú˛úˇ˘¸ß·Ö×Ӻϳɵȹ¤ŇµŁ¬˛ÝËᾧĚĺĘÜČȵ˝100ˇćʱʧȥ˝áľ§Ë®Ł¬łÉÎŞÎŢË®˛ÝËᡣijѧϰС×éµÄͬѧÄâŇÔ¸ĘŐáÔüÎŞÔÁĎÓĂË®˝âˇŞŃő»ŻˇŞË®˝âŃ»·˝řĐĐÖĆȡ˛ÝËᡣ
|
|
 Çë¸úľÝŇÔÉĎĐĹϢ»Ř´đĎÂÁĐÎĘĚ⣺
Çë¸úľÝŇÔÉĎĐĹϢ»Ř´đĎÂÁĐÎĘĚ⣺˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
ÖĐşÍČȵIJⶨĘǸßÖĐÖŘŇŞµÄ¶¨ÁżĘµŃ顣ȡ0.55mol/LµÄNaOHČÜŇş50mLÓë0.25mol/LµÄÁňËá50mLÖĂÓÚÍĽËůĘľµÄ×°ÖĂÖĐ˝řĐĐÖĐşÍČȵIJⶨʵŃ飬»Ř´đĎÂÁĐÎĘĚ⣺
(1)´ÓÉĎͼʵŃé×°ÖĂż´Ł¬ĆäÖĐÉĐȱÉٵÄŇ»ÖÖ˛ŁÁ§ÓĂĆ·ĘÇ_________ _Ł¬łý´ËÖ®Í⣬װÖĂÖеÄŇ»¸öĂ÷ĎÔ´íÎóĘÇ ˇŁ
(2)ÎŞ±ŁÖ¤¸ĂʵŃéłÉą¦¸Ăͬѧ˛ÉȡÁËĐí¶ŕ´ëĘ©Ł¬ČçÍĽµÄËéÖ˝ĚőµÄ×÷ÓĂÔÚÓÚ________ ___ˇŁ
(3)Čô¸ÄÓĂ60mL 0.25molˇ¤L-1 H2SO4şÍ50mL 0.55molˇ¤L-1 NaOHČÜŇş˝řĐĐ·´Ó¦ÓëÉĎĘöʵŃéĎŕ±ČŁ¬Ëů·ĹłöµÄČČÁż Ł¨ĚĎŕµČˇ±ˇ˘ˇ°˛»ĎŕµČˇ±Ł©Ł¬ČôʵŃé˛Ů×÷ľůŐýČ·Ł¬ÔňËůÇóÖĐşÍČČ ĚĎŕµČˇ±ˇ°˛»ĎŕµČˇ±Ł©
(4)µąČëNaOHČÜŇşµÄŐýČ·˛Ů×÷ĘÇŁş________ˇŁ (´ÓĎÂÁĐѡłö)ˇŁ
AŁ®ŃزŁÁ§°ô»şÂýµąČë
BŁ®·ÖČý´ÎÉŮÁżµąČë
CŁ®Ň»´ÎѸËٵąČë
(5)ĘąÁňËáÓëNaOHČÜŇş»ěşĎľůÔȵÄŐýČ·˛Ů×÷ĘÇŁş________ˇŁ (´ÓĎÂÁĐѡłö)ˇŁ
AŁ®ÓĂζȼĆСĐÄ˝Á°č
BŁ®˝ŇżŞÓ˛Ö˝Ć¬ÓòŁÁ§°ô˝Á°č
CŁ®ÇáÇáµŘŐńµ´ÉŐ±
DŁ®ÓĂĚ×ÔÚζȼĆÉϵĻ·ĐβŁÁ§°ôÇáÇáµŘ˝Á¶Ż
(6)ʵŃéĘýľÝČçĎÂ±íŁş
˘ŮÇëĚîдϱíÖеĿհףş
| ÎÂ¶Č ĘµŃé´ÎĘý | ĆđʼζČt1ˇć | ÖŐֹζČt2/ˇć | ζȲîĆ˝ľůÖµ (t2Łt1)/ˇć | ||
| H2SO4 | NaOH | Ć˝ľůÖµ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
ż×ȸʯÖ÷ŇŞş¬Cu2(OH)2CO3Ł¬»ąş¬ÉŮÁżFeµÄŃő»ŻÎďşÍSiO2Ł¬ĘµŃéĘŇŇÔż×ȸʯΪÔÁĎÖƱ¸CuSO4ˇ¤5H2OĽ°CaCO3Ł¬˛˝ÖčČçĎÂŁş 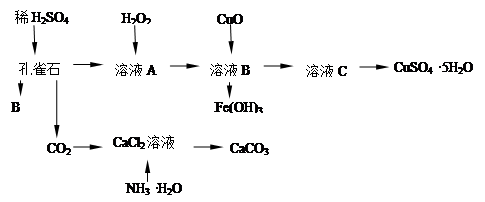
ĘԻشđĎÂÁĐÎĘĚ⣺
Ł¨1Ł©BÎďÖʵĻŻŃ§Ę˝ĘÇ ÔÚδĽÓČëH2O2µÄČÜŇşÖĐŁ¬´ćÔڵĽđĘôŔë×ÓÓĐCu2+ˇ˘Fe2+ˇ˘Fe3+ˇŁČôĽěŃé¸ĂČÜŇşÖĐFe3+Ł¬ŃˇÔń×îşĎĘʵÄĘÔĽÁĘÇ Ł¨Ěî´úşĹŁ©ˇŁ
| AŁ®KMnO4ČÜŇş | BŁ®Fe·Ű | CŁ®Na2CO3ČÜŇş | DŁ®KSCNČÜŇş |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
ÄłNa2CO3ŃůĆ·ÖĐ»ěÓĐŇ»¶¨ÁżµÄNa2SO4 (Éčľů˛»ş¬˝áľ§Ë®Ł©Ł¬Äł»ŻŃ§ĐËȤС×é˛ÉÓĂÁ˝ÖÖ·˝°¸˛â¶¨¸ĂŃůĆ·ÖĐNa2CO3µÄÖĘÁż·ÖĘýŁ¬ĘԻشđĎÂÁĐÎĘĚ⡣
·˝°¸Ň»ŁşŔűÓĂĎÂÁĐ·˝°¸ÍęłÉNa2CO3ÖĘÁż·ÖĘýµÄśy¶¨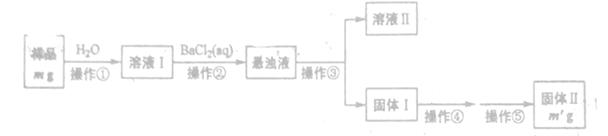
(1)˛Ů×÷˘ŰşÍ˘ÜµÄĂűłĆ·Ö±đÎŞ_______ˇŁ
(2)ÉĎĘö˛Ů×÷˘Ůˇ«˘ÜÖĐŁ¬ĘąÓõ˝˛ŁÁ§°ôµÄÓĐ______(Ěî˛Ů×÷ĐňşĹ)ˇŁ
(3)ĹжϲŮ×÷˘Ú·ńÍęłÉµÄ·˝·¨ĘÇ______
·˝°¸¶ţŁş˛ÉÓĂĎÂͼʵŃé×°ÖĂŁ¨ĽĐłÖŇÇĆ÷ŇŃʡÂÔŁ©.ѡÓĂĎÂÁĐĘÔĽÁ: a.ŨÁňËáb.±ĄşÍNaHCO3ČÜŇşC.6mol/LŃÎËáD.2mol/LÁňËá, e.ĽîĘŻ»Ňf. ÎŢË®CaCl2,śy¶¨ŃůĆ·ÖĐNa2CO3,µÄÖĘÁż·ÖĘýŁş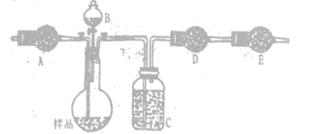
(4)ĚîĐ´±íÖĐżŐ¸ńŁş
| ŇÇĆ÷ | ĘÔĽÁ | ĽÓČë¸ĂĘÔĽÁµÄÄżµÄ |
| A | | ąÄČëżŐĆřʱϴȥCO2 |
| B | | ĘąŃůĆ·łä·Ö·´Ó¦·ĹłöĆřĚĺ |
| C | a | |
| D | e | łä·ÖÎüĘŐCO2 |
| E | e | |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
ĚĽËá¸ĆşÍÁňËá¸Ć¶ĽĘǸƵÄÖŘŇŞ»ŻşĎÎËüĂÇÔÚÉú˛úÉú»îÖжĽÓĐ×Ĺąă·şµÄÓ¦Ó᣼ס˘ŇŇÁ˝×éͬѧ·Ö±đ¶ÔĚĽËá¸ĆµÄÖƱ¸ˇ˘ÁňËá¸ĆµÄĐÔÖĘ˝řĐĐÁËŇÔĎÂĚ˝ľżŁ¬ÇëÄă˛ÎÓ벢ÍęłÉ¶ÔÓĐąŘÎĘĚâµÄ˝â´đˇŁ
(1)Ľ××éĘąÓĂ´óŔíĘŻ(ş¬ÉŮÁżµÄFe2O3ÔÓÖĘ)µČÎďÖĘÖƱ¸ĚĽËá¸ĆµÄʵŃéÁ÷łĚČçĎÂŁş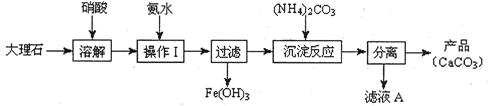
˘ŮČÜ˝â´óŔíʯʱŁ¬ÓĂĎőËá¶ř˛»ÓĂÁňËáµÄÔŇňĘÇ ˇŁ
˘ÚÉĎĘöÁ÷łĚÖĐŁ¬ˇ°·ÖŔ롱µĂ˛úĆ·Ëů°üş¬µÄʵŃé˛Ů×÷ŇŔ´ÎÎŞŁşąýÂˡ˘ ˇ˘ ˇŁ
˘Űˇ°ÂËŇşAˇ±ÖĐłýH+Ŕë×ÓÍ⣬»ąş¬ÓеÄŃôŔë×ÓĘÇ Ł»ĽěŃé¸ĂŃôŔë×ÓµÄʵŃé·˝·¨ĘÇŁşČˇÉŮÁżÂËŇşAÓë ÔÚĘÔąÜÖĐ»ěşĎˇ˘ĽÓČČłä·Ö·´Ó¦Ł¬˝«ĘŞČóµÄşěÉ«ĘŻČďĘÔÖ˝(»ňpHĘÔÖ˝)żż˝üĘԹܿڣ¬ąŰ˛ěĎÖĎ󼴿ɡŁ
(2)ŇŇ×é¶ÔÄłÁňËá¸Ćľ§Ěĺ(xCaS04ˇ¤yH20)ĽÓČČ·Ö˝âµÄÓйط´Ó¦˝řĐĐĚ˝ľżˇŁËűĂÇȡ6.52g¸Ăľ§Ěĺ˝řĐĐĽÓČČŁ¬ĽÓČČąýłĚÖĐŁ¬ąĚĚĺÖĘÁżËćʱĽäµÄ±ä»ŻÇéżöČçĎÂÍĽËůĘľˇŁÓÖÖŞt5ˇ«t6ʱĽä¶ÎÄÚąĚĚĺÖĘÁżĽőÇáµÄÔŇňĘDzúÉúÁËÁ˝ÖÖĆřĚ壬·´Ó¦µÄ»ŻŃ§·˝łĚʽΪŁş
2CasO4  2CaO+2S02ˇü+O2ˇüˇŁ
2CaO+2S02ˇü+O2ˇüˇŁ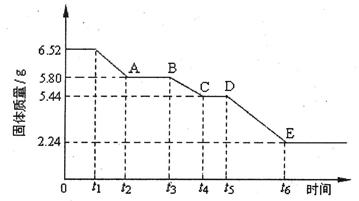
˘ŮĽÓČČʱŁ¬¸Ăľ§Ě忪ʼ·˘Éú»ŻŃ§±ä»ŻµÄʱĽäĘÇ (Ět1ˇ±ˇ˘ˇ°t3ˇ±»ňˇ°t5ˇ±)ˇŁ
˘Út4ˇ«t5ʱĽä¶ÎąĚĚĺµÄ»ŻŃ§Ę˝ÎŞ ˇŁ
˘Űtlˇ«t2ʱĽä¶ÎąĚĚĺ·˘Éú·´Ó¦µÄ»ŻŃ§·˝łĚʽΪ ˇŁ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘµŃéĚâ
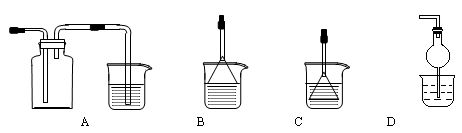
A×°ÖĂÖĐ×°Óе»ĆÉ«µÄąĚĚ壬·ÖҺ©¶·ÖĐ×°ÓĐŨŃÎËᣬBÖĐʢŨÁňËᣬCÖĐ·ĹÓĐ´ß»ŻĽÁŁ¬DÖĐʢµí·Űµâ»ŻĽŘČÜŇşŁ¬EÖĐʢ×ăÁżµÄNaOHČÜŇşŁ¬FÖĐʢFeSO4şÍH2SO4»ěşĎČÜŇşˇŁ 
ĎČ´ňżŞÖąË®ĽĐŁ¬Í¨ČëN2Ł¬´ý×°ÖĂÖĐżŐĆř±»¸ĎľˇşóąŘڼֹˮĽĐŁ¬µăČĽľĆľ«µĆŁş´Ó·ÖҺ©¶··ĹČëŨŃÎËᣬDÖĐČÜҺѸËٱäŔ¶Ł¬FÖĐČÜŇşÓÉÇłÂĚÉ«±äÎŞ×Ř»ĆÉ«(Őű¸ö×°ÖĂąÔO3)ˇŁ
Ł¨1Ł©µ»ĆÉ«ąĚĚĺËůş¬»ŻŃ§ĽüµÄĂűłĆÎŞŁş________Ł»×°ÓĐŐÚ»ĆÉ«µÄąĚĚĺŇÇĆ÷µÄĂűłĆ_______ˇŁ
Ł¨2Ł©ČçşÎĽě˛é×°ÖõÄĆřĂÜĐÔ___________________________________________________ˇŁ
Ł¨3Ł©CÖĐ·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝___________________________________________________ˇŁ
Ł¨4Ł©E×°ÖĂÖĐ·´Ó¦µÄŔë×Ó·˝łĚĘ˝_______________________________________________ˇŁ
Ł¨5Ł©ČçFÖĐČÔÓĐ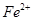 Ł¬ČçşÎĽěŃ麬ÓĐFe2+____________________________________ˇŁ
Ł¬ČçşÎĽěŃ麬ÓĐFe2+____________________________________ˇŁ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşµĄŃˇĚâ
Ö»ÓĂŇ»ÖÖĘÔĽÁĽř±đŐýĽşÍ顢1-ĽşĎ©ˇ˘ŇŇ´Ľˇ˘±˝·ÓË®ČÜŇş4ÖÖÎŢÉ«ŇşĚ壬ӦѡÓĂ
| AŁ®ËáĐÔKMnO4ČÜŇş | BŁ®±ĄşÍäĺË® | CŁ®NaOHČÜŇş | DŁ®AgNO3ČÜŇş |
˛éż´´đ°¸şÍ˝âÎö>>
°Ů¶ČÖÂĐĹ - Á·Ď°˛áÁбí - ĘÔĚâÁбí
şţ±±Ęˇ»ĄÁŞÍřÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨Ć˝Ě¨ | ÍřÉĎÓĐş¦ĐĹϢľŮ±¨×¨Çř | µçĐĹթƾٱ¨×¨Çř | ÉćŔúĘ·ĐéÎŢÖ÷ŇĺÓĐş¦ĐĹϢľŮ±¨×¨Çř | ÉćĆóÇÖȨľŮ±¨×¨Çř
ÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨µç»°Łş027-86699610 ľŮ±¨ÓĘĎ䣺58377363@163.com