
����Ŀ�������Լ���RMgX�����л���Ӧ�е�һ����Ҫ�Լ��������Ʒ�Ϊ�� ��RΪ������XΪ±�أ���
��RΪ������XΪ±�أ���
������ϩ���ʵ�������ϳ������춡��![]() �Ĺ������£���Ӧ����û���г�����
�Ĺ������£���Ӧ����û���г�����

�Իش��������⣺
��1�����������У����ڣ���������ӳɷ�Ӧ����________________________������ţ���
��2��A�����������ŵ�������____________��C�����������ŵ�������____________��
��3����ϵͳ��������F��������������________________________��
��4��д��E�Ľṹ��ʽ________________________��
��5����Ӧ�ڵĻ�ѧ����ʽ____________________________________��
��6����Ӧ�ߵĻ�ѧ����ʽ____________________________________��
��7��B��������Һ��Ӧ�ķ���ʽ____________________________________��
��8��д��C��������ͬ����л�����칹��____________��____________��
���𰸡� �٢ܢ� �ǻ� �Ȼ� 2-���� CH3CH2MgX 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CH2CH��OH��CH3+CH3COOH
2CH3CHO+2H2O CH3CH2CH��OH��CH3+CH3COOH![]() CH3COOCH(CH3)CH2CH3+H2O CH3CHO+2Ag��NH3��2OH
CH3COOCH(CH3)CH2CH3+H2O CH3CHO+2Ag��NH3��2OH![]() CH3COONH4+3NH3��+2Ag+H2O HCOOCH3 HOCH2CHO
CH3COONH4+3NH3��+2Ag+H2O HCOOCH3 HOCH2CHO
����������ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���������������BΪCH3CHO����ȩ��һ������������Ӧ����CΪCH3COOH����ϩ��HX�����ӳɷ�Ӧ����DΪCH3CH2X��D��Mg�����������·�����Ӧ����EΪCH3CH2MgX��E����ȩ�����ӳɷ�Ӧ��ˮ������FΪCH3CH2CH��OH��CH3�������ᷢ��������Ӧ�õ������춡������
��1���������Ϸ�����֪���������У����ڣ���������ӳɷ�Ӧ���Ǣ٢ܢޡ���2��A���Ҵ����������������ŵ��������ǻ���C�����ᣬ�������������ŵ��������Ȼ�����3��FΪCH3CH2CH��OH��CH3����������2-��������4��E�Ľṹ��ʽΪCH3CH2MgX����5����Ӧ�����Ҵ�����������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����6����Ӧ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3CH2CH��OH��CH3+CH3COOH
2CH3CHO+2H2O����6����Ӧ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3CH2CH��OH��CH3+CH3COOH![]() CH3COOCH(CH3)CH2CH3+H2O����7��B��������Һ��Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH
CH3COOCH(CH3)CH2CH3+H2O����7��B��������Һ��Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH![]() CH3COONH4+3NH3��+2Ag+H2O����8��C�����ᣬC��������ͬ����л�����칹��ΪHCOOCH3��HOCH2CHO��
CH3COONH4+3NH3��+2Ag+H2O����8��C�����ᣬC��������ͬ����л�����칹��ΪHCOOCH3��HOCH2CHO��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�п��ܺ��д�����Mg2����Al3����H����Cl��������OH���������Һ����μ���0.5 mol��L��1NaOH��Һ�����ɳ����������ͼ���NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ������ж�ԭ��Һ��(����)
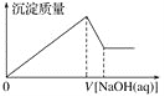
A. ��Mg2����û��Al3��
B. ��Al3����û��Mg2��
C. ��Mg2����Al3��
D. �����H����Mg2����Al3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a��b�����������������Ԫ�أ�c�ǵؿ��к�������Ԫ�أ�d��bͬ�壬e2+���ӵ�3d�������9�����ӡ��ش��������⣺
��1��c��d����Ԫ���γɵĻ�����ͳ�ƹ�ʯ����ͨ��______________����������ᾧ�κ����ε����ִ�����̬��c�ĵ����Ų�ͼΪ_______________________��
��2��A��B�����������ֳ������л��A����CaCO3��Ӧ�������ڳ�����ˮ����B�����е�̼ԭ����Ŀ��A����ͬ���ɷ���������Ӧ��A�д��ڻ�ѧ����������______��
A.���Ӽ� B.���Լ� C.�Ǽ��Լ� D.�Ҽ� E.�м�
B���ӹ�������̼ԭ�ӵĹ���ӻ�������____��
��3���á�>����<����գ�
��һ������ | �۵� |
b___d | dc2����___d���� |
��4��c��e����Ԫ�ؿ��γ�һ�ְ뵼����ϣ���ѧʽΪe2c���������������ڲ����ĸ�cԭ�ӣ�����cԭ��λ�����ĺͶ��㣬��þ�������____��eԭ�ӣ�eԪ��λ��Ԫ�����ڱ���_______����
��5����e2+�����ε�ˮ��Һ�м�������İ�ˮ���ɵõ�����ɫ����Һ�������Ҵ�����������ɫ���壬����д������������ӷ���ʽ___________________��
��6��e����Ϊ�����������壬��ԭ�Ӱ뾶Ϊrcm���侧���ⳤΪa nm����e���ʵ��ܶ�Ϊ__________g��cm-3����ռ������ʵļ���ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾʵ��װ������֤���ȶ���Na2CO3ǿ��NaHCO3����ش�:
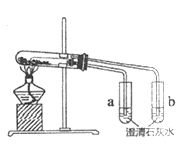
(1) NaHCO3���ȷֽ�Ļ�ѧ����ʽ��_________��
(2)֤�����ȶ���Na2CO3ǿ��NaHCO3��ʵ��������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ӹ�ҵ����FeCl3��Һ��ʴ��Ե���ϵ�ͭ��������ӡˢ��·�塣�Ӹ�ʴ��Һ(��Ҫ��FeCl3��FeCl2��CuCl2 )�л���ͭ�������»��FeCl3��Һ����Һ�����������£�
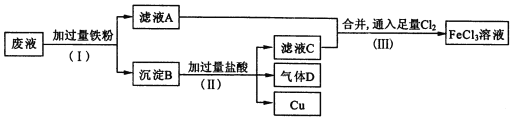
��1������(��)���������� ��____________��
��2������B����Ҫ����_________������D��______________��
��3��д������(��)������FeCl3�Ļ�ѧ����ʽ____________________��
��4������(��)�У�����������H2O2Ҳ�ܴﵽͬ����Ŀ�ģ�д��H2O2��Fe2+����ΪFe3+�����ӷ���ʽ:_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Һ�п��Դ��������һ���ǣ���
A. H+ Na+ OH�� B. Na+ NO3�� Cl��
C. K+ H+ HCO3�� D. Ca2+ SO42�� CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ӵ�ع���ԭ������ͼ��ʾ����ط�ӦΪ��Li1��xCoO2��LixC![]() LiCoO2��C������˵������ȷ����
LiCoO2��C������˵������ȷ����

A. �ŵ�ʱ�����Ӵ�b�����õ�������a��
B. �ŵ�ʱ����ת��1mol e����̼���Ͻ�����7 g
C. ���ʱ�������ͨ����Ĥ��������
D. ���ʱ��a����Ӧ��LiCoO2��xe��= Li1-xCoO2��xLi+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ҫ����������Ʒ֮һ���ڹ�����ռ����Ҫ��λ���ҹ���������ý(����Ϊ���Ļ����)�������ϳɰ����ش�����������
��1��Fe��̬ԭ�Ӻ�������Ų�ʽΪ____����������һ����Ҫ���������������ˮ����¶�ڳ�ʪ�����п��ͷų�������Ԫ��Fe��N�У���һ�����ܽϴ����_____����̬ԭ�Ӻ���δ�ɶԵ������϶����_______��
��2��N����������N2O����N2O��CO2��Ϊ�ȵ����壬N2O�Ŀռ乹��Ϊ__________��
��3��N�ж����⻯�������(N2H4)����������ƽ�����ȼ�ϣ�N2H4��Nԭ�ӵ��ӻ���ʽΪ____��
��4��N��P��AsΪͬ��Ԫ�أ�NH3��PH3��AsH3�������ʵķе��ɸߵ��͵�˳��Ϊ_____��ԭ����____��
��5��K3[Fe(CN)6]�����ڼ���Fe2����K3[Fe(CN)6]�д��ڵĻ�ѧ��������_______��
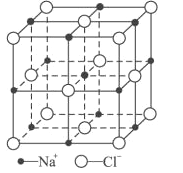
��6��FeO����ľ�����NaCl�����ƣ�NaCl�ľ�����ͼ��ʾ�����ھ���ȱ�ݣ�ij�������������ʵ�����ΪFe0.9O�����а�����Fe2����Fe3���������߳�Ϊ428pm����þ�����ܶ�Ϊ____g/cm3(�г�����ʽ���ɣ���NA��ʾ�����ӵ�������ֵ)��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������2Fe2����Cl2��2Fe3����Cl����2Fe3����Cu��2Fe2����Cu2����Fe��Cu2����Fe2����Cu������Ӧ�ж���������������ǿ��˳����ȷ����
A.Cl2��Fe3����Cu2��B.Fe3����Cl2��Cu2��
C.Cu2����Fe3����Cl2D.Cl2��Cu2����Fe3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com