
”¾ĢāÄæ”æĮņ¼°Ęä»ÆŗĻĪļŌŚÉś²śÉś»īÖŠÓ¦ÓĆ¹ć·ŗ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Ģśļ§·ÆŹĒŅ»ÖÖ»ÆѧĪļÖŹ,·Ö×ÓŹ½ŹĒ NH4Fe(SO4)2”¤12H2O,ĘäČÜÓŚĖ®ŗó,ČÜŅŗÖŠµÄĄė×ÓÅØ¶Č“óŠ”¹ŲĻµĪŖ__________________”£
(2)ŅŃÖŖijČÜŅŗÖŠŗ¬ÓŠ CO32£”¢SO42£µČĄė×Ó,Č”Ņ»¶ØĮæµÄøĆČÜŅŗ,ĻņĘäÖŠµĪ¼ÓBaCl2ČÜŅŗ£¬µ± CO32£æŖŹ¼³Į µķŹ±£¬ČÜŅŗÖŠc(CO32-)/c(SO42-)ĪŖ_______________”£(ŅŃÖŖ Ksp(BaSO4 )£½1.0”Į10£10 £¬Ksp(BaCO3)£½2.5”Į10£9 )
(3)ŅŃÖŖ£ŗS2Cl2(l)£«Cl2(g)£½2SCl2(l) ¦¤H£½£50.2kJ”¤mol£1 ”£¶ĻĮŃ 1molCl£Cl¼ü”¢1molS£S¼ü·Ö±šŠčŅŖĪüŹÕ 243kJ”¢268kJ µÄÄÜĮ棬Ōņ¶ĻĮŃ 1mol S£Cl¼üŠčŅŖĪüŹÕµÄÄÜĮæĪŖ____kJ”£
(4)ÓĆ NaOH ČÜŅŗĪüŹÕŃĢĘųÖŠµÄ SO2,½«ĖłµĆµÄ Na2SO3 ČÜŅŗ½ųŠŠµē½ā,æÉŅŌÖʱøH2SO4£¬ĘäŌĄķČēĻĀĶ¼ĖłŹ¾(µē¼«²ÄĮĻĪŖŹÆÄ«)”£
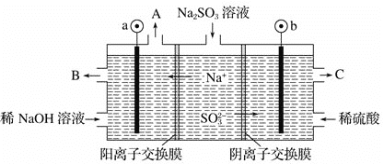
Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ______________________£»ĘäÖŠæÉŃ»·Ź¹ÓƵÄĪļÖŹŹĒ________”£
”¾“š°ø”æc(SO42£)>c(NH4£«)>c(Fe3£«)>c(H£«)>c(OH£) 25 280.6 SO32££2e££«H2O=SO42££«2H£« NaOH
”¾½āĪö”æ
£Ø1£©ĮņĖįĢśļ§NH4Fe(SO4)2”¤12H2OČÜÓŚĖ®ŠĪ³ÉµÄČÜŅŗÖŠ£¬Fe3£«ŗĶNH4£«Ė®½āČÜŅŗĻŌĖįŠŌ£¬Fe3£«µÄĖ®½ā³Ģ¶Č“óÓŚNH4£«£¬Ė®½ā³Ģ¶ČŌ½“ó£¬Ąė×ÓÅضČŌ½Š”£¬¾Ż“Ė½ā“š£»
£Ø2£©øł¾Żc(CO32-)/c(SO42-)= Ksp(BaCO3)/ Ksp(BaSO4 )¼ĘĖć£»
£Ø3£©·“Ó¦µÄģŹ±äµČÓŚ·“Ó¦ĪļµÄ¼üÄܼõČ„Éś³ÉĪļµÄ¼üÄÜ£¬Éč¶ĻĮŃ1mol S£Cl¼üŠčŅŖĪüŹÕµÄÄÜĮæĪŖx£¬ĮŠ·½³ĢĒó½ā£»
£Ø4£©Ķ¼ÖŠŅõĄė×ÓĻņb¼«ŅĘ¶Æ£¬ŌņbĪŖŃō¼«£¬ĖłŅŌb¼«ÉĻSO32£ŌŚŃō¼«Ź§µē×ÓÉś³ÉSO42££¬¾Ż“Ė½ā“š”£
£Ø1£©ĮņĖįĢśļ§NH4Fe(SO4)2”¤12H2OČÜÓŚĖ®ŠĪ³ÉµÄČÜŅŗÖŠ£¬Fe3£«ŗĶNH4£«Ė®½āČÜŅŗĻŌĖįŠŌ£¬Fe3£«µÄĖ®½ā³Ģ¶Č“óÓŚNH4£«£¬Ė®½ā³Ģ¶ČŌ½“ó£¬Ąė×ÓÅضČŌ½Š”£¬ĖłŅŌc(NH4£«)>c(Fe3£«)£¬ĮņĖįøłĄė×Ó²»Ė®½ā£¬ÅضČ×ī“ó£¬ĖłŅŌČÜŅŗÖŠĄė×ÓÅØ¶Č¹ŲĻµc(SO42£)>c(NH4£«)>c(Fe3£«)>c(H£«)>c(OH£)”£
Ņņ“Ė£¬±¾ĢāÕżČ·“š°øŹĒ£ŗc(SO42£)>c(NH4£«)>c(Fe3£«)>c(H£«)>c(OH£)£»
£Ø2£©øł¾Żc(CO32-)/c(SO42-)= Ksp(BaCO3)/ Ksp(BaSO4 )æÉÖŖ£¬c(CO32-)/c(SO42-)=![]() 25”£
25ӣ
Ņņ“Ė£¬±¾ĢāÕżČ·“š°øŹĒ£ŗ25£»
£Ø3£©·“Ó¦µÄģŹ±äµČÓŚ·“Ó¦ĪļµÄ¼üÄܼõČ„Éś³ÉĪļµÄ¼üÄÜ£¬Éč¶ĻĮŃ1mol S£Cl¼üŠčŅŖĪüŹÕµÄÄÜĮæĪŖx£¬Ōņ2x+268 kJ+243 kJ-4x=-50.2 kJ£¬½āµĆx=280.6 kJ”£
Ņņ“Ė£¬±¾ĢāÕżČ·“š°øŹĒ£ŗ280.6£»
£Ø4£©Ķ¼ÖŠŅõĄė×ÓĻņb¼«ŅĘ¶Æ£¬ŌņbĪŖŃō¼«£¬ĖłŅŌb¼«ÉĻSO32£ŌŚŃō¼«Ź§µē×ÓÉś³ÉSO42££¬Ęäµē¼«·½³ĢŹ½ĪŖ£ŗSO32££2e££«H2O=SO42££«2H£«£»aĪŖŅõ¼«£¬Ņõ¼«Ēų·ÅµēĄė×ÓĪŖĒāĄė×ÓÉś³ÉĒāĘų£¬BæŚĮ÷³öµÄĪļÖŹŹĒÅØ¶Č½Ļ“óµÄĒāŃõ»ÆÄĘČÜŅŗ£¬æÉÓĆĄ“ĪüŹÕ¶žŃõ»ÆĮņ£¬¹ŹæÉŃ»·Ź¹ÓƵÄĪļÖŹŹĒNaOH£¬
Ņņ“Ė£¬±¾ĢāÕżČ·“š°øŹĒ£ŗSO32££2e££«H2O=SO42££«2H£« £»NaOH”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¹ŲÓŚ·Ö×Ó”¢Ō×Ó”¢Ąė×ÓµÄĖµ·Ø£¬“ķĪóµÄŹĒ£Ø £©
¢Ł¶žŃõ»ÆĢ¼ÓÉĢ¼Ō×ÓŗĶŃõŌ×Ó¹¹³É£»¢ŚµČÖŹĮæµÄŃõĘųŗĶŅŗŃõ£¬ŃõĘųĢå»ż±ČŅŗŃõ“ó£¬ĖµĆ÷·Ö×Ó¼äĻ¶·¢ÉśĮĖøı䣻¢Ū·Ö×ÓŹĒ±£³ÖĪļÖŹŠŌÖŹµÄŅ»ÖÖĪ¢Į££»¢Ü·Ö×ÓŅ»¶Ø±ČŌ×ӓ󣻢ŻŌ×ÓŹĒ×īŠ”µÄĮ£×Ó£¬²»æÉŌŁ·Ö
A.¢Ł¢Ū¢Ü¢ŻB.¢Ś¢Ū¢Ü¢Ż
C.¢Ł¢Ś¢Ū¢Ü¢ŻD.¢Ł¢Ū¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÄ³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼ĘĮĖŅ»×鏵ŃéŃéÖ¤ŌŖĖŲÖÜĘŚĀÉ£®
¢ń£®¼×Ķ¬Ń§Éč¼ĘĮĖČēĻĀĶ¼×°ÖĆĄ“Ņ»“ĪŠŌĶź³ÉŌŖĖŲµŖ”¢Ģ¼”¢¹č·Ē½šŹōŠŌĒæČõµÄ±Č½Ļ”£
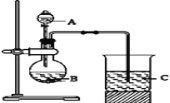
£Ø1£©¼×Ķ¬Ń§ŌŚĮ¬½ÓŗĆŅĒĘ÷ŗ󣬼ÓČėŅ©Ę·Ö®Ē°ŅŖ¼ģ²é×°ÖĆĘųĆÜŠŌ”£Ź×ĻČ¹Ų±Õ_____£¬½«µ¼¹ÜÉģČėÉÕ±ŅŗĆęŅŌĻĀ£¬ŌŁ_____£¬Čē¹ūCÖŠ______£¬ŌņĖµĆ÷________
£Ø2£©ŅŖÖ¤Ć÷µŖ”¢Ģ¼”¢¹č·Ē½šŹōŠŌĒæČõ£¬ŌŚAÖŠ¼Ó________ČÜŅŗ£¬BÖŠ¼Ó____ČÜŅŗ£¬CÖŠ¼Ó________ČÜŅŗ£¬½«¹Ū²ģµ½CÖŠ__________µÄĻÖĻó£®µ«ĄĻŹ¦ČĻĪŖ£¬øĆĻÖĻó²»×ćŅŌÖ¤Ć÷ČżÕß·Ē½šŹōŠŌĒæČõ£¬ĒėÓĆĪÄ×ÖŠšŹöĄķÓÉ_______”£
£Ø3£©ĪŖ±ÜĆāÉĻŹöĪŹĢā£¬Ó¦ŌŚB”¢CÖ®¼äŌö¼ÓŅ»øöŹ¢ÓŠ×ćĮæ____£ØŃ”ĢīĻĀĮŠ×ÖÄø£ŗA£®ÅØŃĪĖį B£®ÅØNaOHČÜŅŗC£®±„ŗĶNa2CO3ČÜŅŗ D£®±„ŗĶNaHCO3ČÜŅŗ£©µÄĻ“Ęų×°ÖĆ£®øĽųŗóCÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ__________ £®
¢ņ£®±ūĶ¬Ń§Éč¼ĘĮĖČēĻĀĶ¼×°ÖĆĄ“ŃéÖ¤Ā±×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ£®A”¢B”¢CČż“¦·Ö±šŹĒÕ“ÓŠNaBrČÜŅŗµÄĆŽ»Ø”¢ŹŖČóµķ·ŪKIŹŌÖ½”¢ŹŖČóŗģÖ½£®
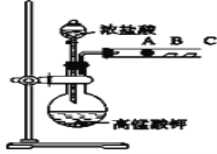
£Ø1£©ĒėŠ“³öÅØŃĪĖįÓėøßĆĢĖį¼Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________
£Ø2£©AÖŠĆŽ»ØŃÕÉ«±ä_______£¬ŌņĖµĆ÷·Ē½šŹōŠŌCl£¾Br£»ĻņNaBrŗĶKIµÄ»ģŗĻČÜŅŗÖŠ£¬ĶØČė×ćĮæµÄCl2³ä·Ö·“Ó¦ŗ󣬽«ĖłµĆČÜŅŗÕōøɲ¢×ĘÉÕ£¬×īŗóµĆµ½µÄĪļÖŹŹĒ_________
£Ø3£©±ūĶ¬Ń§ĄūÓĆ“ĖŹµŃéÖ¤Ć÷Ā±ĖŲµ„ÖŹŃõ»ÆŠŌ£ŗCl2£¾Br2£¾I2£¬ÄćČĻĪŖŗĻĄķĀš____£¬£ØĢī”°ŗĻĄķ”±»ņ”°²»ŗĻĄķ”±£©ĄķÓÉŹĒ___________ £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¹ŲÓŚ¹č¼°Ęä»ÆŗĻĪļµÄŠšŹö“ķĪóµÄŹĒ
A.SiO2ŗÜĪČ¶Ø£¬ÓėĖłÓŠµÄĖį¶¼²»·“Ó¦B.Ė®¾§”¢Āźč§µÄÖ÷ŅŖ³É·Ö¶¼ŹĒSiO2
C.ĢÕ“É”¢²£Į§”¢Ė®Äą¶¼ŹĒ¹čĖįŃĪ²śĘ·D.Ė®²£Į§ŹĒŅ»ÖÖ³£ÓƵÄæóĪļ½ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”¢B”¢C”¢D”¢E”¢F”¢GĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ”£B”¢C”¢D¾łÄÜÓėAŠĪ³É10µē×Ó·Ö×Ó£¬Eµ„ÖŹæÉÓĆÓŚŅ°Ķāŗø½ÓøÖ¹ģµÄŌĮĻ£¬FÓėDĶ¬Ö÷×唣
(1)D”¢E”¢FµÄĄė×Ó°ė¾¶Óɓ󵽊”µÄĖ³ŠņĪŖ_______________________(ĢīĄė×Ó·ūŗÅ)”£
(2)FŗĶGŠĪ³ÉµÄŅ»ÖÖ»ÆŗĻĪļ¼×ÖŠĖłÓŠŌ×Ó¾łĪŖ8µē×ÓĪČ¶Ø½į¹¹£¬øĆ»ÆŗĻĪļÓėĖ®·“Ӧɜ³ÉFµ„ÖŹ”¢FµÄ×īøß¼Ūŗ¬ŃõĖįŗĶGµÄĒā»ÆĪļ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________”£
(3)CÄÜ·Ö±šÓėAŗĶD°“Ō×ÓøöŹż±Č1:2ŠĪ³É»ÆŗĻĪļŅŅŗĶ±ū£¬ŅŅµÄ½į¹¹Ź½ĪŖ__________”£³£ĪĀĻĀ,ŅŗĢåŅŅÓėĘųĢå±ū·“Ӧɜ³ÉĮ½ÖÖĪŽĪŪČ¾µÄĪļÖŹ,øĆ·“Ó¦µÄŃõ»Æ²śĪļÓė»¹Ō²śĪļµÄĪļÖŹµÄĮæÖ®±ČĪŖ________________”£
(4)ĻÖČ”100 mL1 mol/LµÄEµÄĀČ»ÆĪļČÜŅŗ,ĻņĘäÖŠ¼ÓČė1 mol/L NaOHČÜŅŗ²śÉśĮĖ3.9g³Įµķ£¬Ōņ¼ÓČėµÄNaOHČÜŅŗĢå»żæÉÄÜĪŖ_________________________mL”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¶ŌÓŚĘ½ŗāĢåĻµmA(g)£«nB(g)![]() pC(g)£«qD(g) ¦¤H£¼0”£ĻĀĮŠ½įĀŪÖŠ“ķĪóµÄŹĒ( )
pC(g)£«qD(g) ¦¤H£¼0”£ĻĀĮŠ½įĀŪÖŠ“ķĪóµÄŹĒ( )
A. ČōĘ½ŗāŹ±£¬A”¢BµÄ×Ŗ»ÆĀŹĻąµČ£¬ĖµĆ÷·“Ó¦æŖŹ¼Ź±£¬A”¢BµÄĪļÖŹµÄĮæÖ®±ČĪŖm”Ćn
B. ČōĪĀ¶Č²»±ä£¬½«ČŻĘ÷µÄĢå»żĖõŠ”µ½ŌĄ“µÄŅ»°ė£¬“ļµ½ŠĀĘ½ŗāŹ±AµÄÅضČĪŖŌĄ“µÄ1.8±¶£¬Ōņm£«n > p£«q
C. Čōm£«n = p£«q£¬ŌņĶłŗ¬ÓŠa molĘųĢåµÄĘ½ŗāĢåĻµÖŠŌŁ¼ÓČėa molµÄB£¬“ļµ½ŠĀĘ½ŗāŹ±£¬ĘųĢåµÄ×ÜĪļÖŹµÄĮæµČÓŚ2a
D. ČōĪĀ¶Č²»±äĖõŠ”ČŻĘ÷Ģå»ż£¬“ļµ½ŠĀĘ½ŗāŹ±Ń¹ĒæŌö“óµ½ŌĄ“µÄ2±¶£¬ŌņĢå»żŅ»¶ØŠ”ÓŚŌĄ“µÄ1/2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÄ³ŃŠ¾æŠŌѧĻ°Š”×éĄūÓĆŅŌĻĀ×°ÖĆĢ½¾æĀČĘųŗĶ°±ĘųÖ®¼äµÄ·“Ó¦Ēéæö”£ĘäÖŠA”¢F·Ö±šĪŖ°±ĘųŗĶĀČĘųµÄÖĘČ”·¢Éś×°ÖĆ£¬CĪŖ“æ¾»øÉŌļµÄĀČĘųÓėøÉŌļµÄ°±Ęų·¢Éś·“Ó¦µÄ×°ÖĆ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)ŹµŃéŹŅĶس£ÓĆĄ“ÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½ĪŖ ________________________£»
(2)ŹµŃéŹŅŹÕ¼ÆÖʱøµĆµ½µÄ°±ĘųµÄ³£ÓĆ·½·ØŹĒ______________________”£
(3)ĒėĪŖB“¦ŠéĻßæņÄŚŃ”ŌńæÉÄÜŗĻŹŹµÄ×°ÖĆŅŌ¼°ĻąÓ¦µÄŹŌ¼ĮĆū³Ę______ĢīŠņŗÅ”£
A.ĒņŠĪøÉŌļ¹Ü×°¼īŹÆ»Ņ B.Ļ“ĘųĘæ×°ÅØĮņĖį
C.ĒņŠĪøÉŌļ¹Ü×°Ńõ»ÆøĘ D.ĒņŠĪøÉŌļ¹Ü×°ĒāŃõ»ÆÄĘ
(4)×°ÖĆCÄŚ³öĻÖÅØŗńµÄ°×ŃĢ²¢ŌŚČŻĘ÷ÄŚ±ŚÄż½į£¬ĮķŅ»Éś³ÉĪļŹĒæÕĘųµÄÖ÷ŅŖ³É·ÖÖ®Ņ»ĒėŠ“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ __________________ČōÓŠ1molNH3±»Ńõ»Æ£¬Ōņ×ŖŅʵĵē×ÓŹżÄæĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijæĪĢā×éÉč¼ĘŹµŃé¼ģŃéÉśĢśÓėÅØĮņĖį·“Ó¦µÄ²śĪļ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ń.Éč¼ĘČēĻĀ·½°ø£¬¼ģŃéĘųĢå²śĪļÖŠµÄCO2”¢SO2ŗĶH2O”£
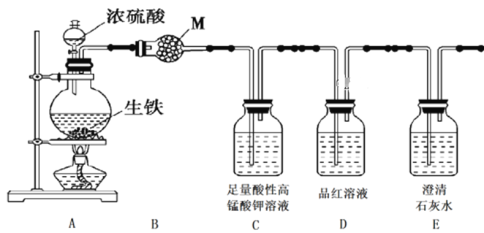
£Ø1£©øÉŌļ¹ÜBÖŠMµÄ»ÆѧŹ½ŹĒ______________________£»
£Ø2£©C×°ÖƵÄ×÷ÓĆŹĒ_________________________________________________£»
£Ø3£©ÄÜÖ¤Ć÷ÓŠ¶žŃõ»ÆĢ¼Éś³ÉµÄŹµŃéĻÖĻóŹĒ_______________________________£»
£Ø4£©“żÉśĢśÓėÅØĮņĖį·“Ó¦Ķź±Ļŗ󣬼ģŃéÉÕĘæÖŠµÄČÜŅŗŗ¬ÓŠFe3£«µÄŹŌ¼ĮŹĒ£ŗ________________”£
¢ņ.øĆæĪĢā×éĶ¬Ń§ČĻĪŖÉśĢśÓėÅØĮņĖį·“Ó¦æÉÄÜÓŠĒāĘųÉś³É”£ĪŖĮĖŃéÖ¤ÕāŅ»²ĀĻė£¬Ń”ŌńĻĀĮŠŅĒĘ÷ŗĶŅ©Ę·²¢½įŗĻÉĻŹö×°ÖĆ£¬ÖŲŠĀÉč¼ĘŹµŃ锣
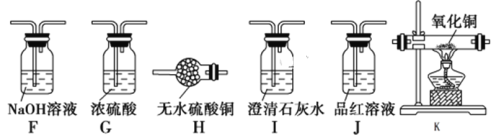
£Ø5£©øł¾ŻŹµŃéÉč¼ĘµÄ¼ņŌ¼ŠŌŌŌņ£¬ĘųĮ÷“Ó×óÖĮÓŅ£¬ŅĒĘ÷ÅÅŠņĪŖ______________”£
£Ø6£©ÄÜÖ¤Ć÷ÓŠĒāĘųÉś³ÉµÄŹµŃéĻÖĻóŹĒ___________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠŹµŃéÉč¼Ę·½°øŅ»¶ØæÉŠŠµÄŹĒ£Ø £©
A.ÓĆĖ®¼ų±š±½ŗĶĖÄĀČ»ÆĢ¼
B.ÓĆ½µĪĀ½į¾§µÄ·½·Ø³żČ„KNO3ÖŠ»ģÓŠÉŁĮæµÄNaCl
C.ÓĆ·ÖŅŗµÄ·½·Ø·ÖĄėµāµÄĖÄĀČ»ÆĢ¼ČÜŅŗ
D.ÓĆ¾Ę¾«ŻĶČ”µāĖ®ÖŠµÄµā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com