
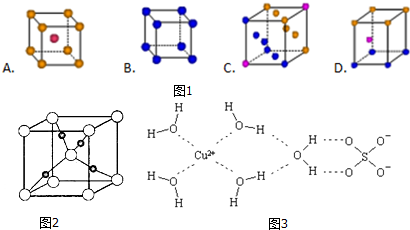
 ��
������ ��1��Cu��ԭ������Ϊ29������������ԭ����д�����Ų�ʽ��CuΪ���ܶѻ�����λ��Ϊ12��
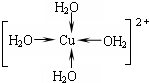
��2��CO��N2Ϊ�ȵ����壬����N2�Ľṹ�жϣ�
��3���ټ״��к���������е�ϸߣ�����ԭ���γ�3���ļ���
�ڸ��ݼ۲���ӶԻ���ģ���жϣ������е���Ϊ�ļ���˫����һ���ļ���һ��Ϊ�м���
�������ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ����Դ��ж�ͭԭ������
��4����Cu2+�ṩ�չ����ˮ����ԭ���ṩ�¶Ե��ӣ��γ���λ����
�������һ����Ӽ�������ǿ��Ӱ�����ʵ��������ʣ�
��� �⣺��1����Cu��ԭ������Ϊ29�������Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1����CuΪ���ܶѻ�����λ��Ϊ12��C���ϣ�
�ʴ�Ϊ����1s22s22p63s23p63d104s1��[Ar]3d104s1����C��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪ��N��N����Ϊ�ȵ�������ӵĽṹ���ƣ���д��CO�ĽṹʽΪC��O��
�ʴ�Ϊ��C��O��
��3���ټ״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ���ȩ�����к���̼��˫������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ����ʴ�Ϊ���״�����֮���γ������sp2�ӻ���
�ڼ�ȩΪsp2�ӻ��������µ��Ӷԣ����ӵĿռ乹��Ϊƽ�������Σ�1mol��ȩ�����к���2mol̼��ļ���1mol̼���ļ����ʺ��Цļ������ʵ���Ϊ3mol����ĿΪ3NA����
�ʴ�Ϊ��ƽ�������Σ�3NA��
�����ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ�����������Cuԭ����ĿΪ4��
�ʴ�Ϊ��4��
��4����Cu2+�ṩ�չ����ˮ����ԭ���ṩ�¶Ե��ӣ��γ���λ����ˮ��ͭ���ӵĽṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
�������һ����Ӽ�������ǿ������ˮ���ۡ��е�ϸߣ�����������з����ԣ����ʱ��������࣬��������ܶȼ�С��
�ʴ�Ϊ��ˮ���ۡ��е�ϸߣ����ʱ�ܶȼ�С��
���� �����ԭ�ӽṹ�����ӽṹ������ṹ�������֪ʶ�Ϻõ��ں���һ���ۺϿ��������ʽṹ�����ʵ�����֪ʶ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�������£�Cl2���ڼױ��ı���������Ϸ���ȡ����Ӧ | |
| B�� | ������2��2����������Br2��Ӧ����һ��ȡ����ֻ��һ�� | |
| C�� | ����ͱ�ϩ�����ʵ�����1 mol����ȫȼ������3 mol H2O | |
| D�� | ����������Һ��ͨ��CO2���ɴ����ᣬ��̼������Աȴ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
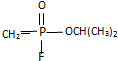 ����֪
����֪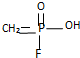 ������Ϊ�����ᣬ��ɳ�֡��Ļ�ѧ����Ϊ��������
������Ϊ�����ᣬ��ɳ�֡��Ļ�ѧ����Ϊ��������| A�� | ����������� | B�� | ����������� | ||
| C�� | ������������� | D�� | ������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ӧ����Թ��ð�ˮϴ�� | |
| B�� | ��������ʵ�����ձ��þƾ�ϴ�� | |
| C�� | ʢװ���Ӻ���Թ�������ϴ�� | |
| D�� | �ռ��������������Թ���̼������Һϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�ӽṹʾ��ͼ��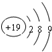 | B�� | CCl4�ĵ���ʽ�� | ||
| C�� | HClO�Ľṹʽ��H-Cl-O | D�� | MgCl2�ĵ���ʽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
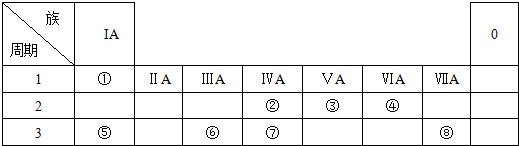
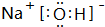
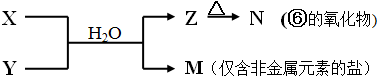
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
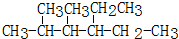
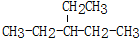 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����һ�������NaCl��Һ | B�� | ����һ�������KNO3������Һ | ||
| C�� | ����������Na2CO3���� | D�� | ����������CuSO4���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com