
| ʵ�� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L����״���� |
| 1 | 7.24 | 50.00 | 1.792 |
| 2 | 14.48 | 50.00 | 3.584 |
| 3 | 21.72 | 50.00 | 4.032 |
| 4 | 36.20 | 50.00 | 2.240 |
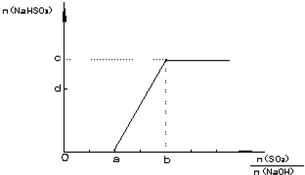
| a | ||
36.2-
|
| 2 |
| 1 |
(0.2+
| ||
| 0.05L |
| a | ||
36.2-
|
| 2 |
| 1 |
(0.2+
| ||
| 0.05L |
| 1 |
| 2 |
| 1 |
| 2 |


 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L |
�� | 7.4 | 40.00 | 1.68 |
�� | 14.8 | 40.00 | 3.36 |
�� | 22.2 | 40.00 | 1.12 |
�� | 37.0 | 40.00 | 0.00 |
��1��ʵ��������йط�Ӧ�����ӷ���ʽΪ��________________________________��
��2���ɢ�������ֱ���Ʋ⣺��״������3.7 g��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ________L��
��3���Լ���û�����еģ�NH4��2SO4��NH4HSO4���ʵ���֮��________��
��4���������NaOH��Һ�����ʵ���Ũ�ȣ�Ӧѡ���________�����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ�����и����������ʼ죨���ۣ���ѧ���� ���ͣ������
��ҵ����������Ϊ��Ҫԭ�ϳ�ȡ���ᣬ��Ҫ�豸�з����ף��Ӵ��Һ���������
��1���������ڽ������¯ǰ��Ҫ���飬��Ŀ���� ��
��2��Ϊ�˳�����÷�Ӧ�ų����������Ӵ�����Ӧ��װ �����豸���ƣ������������������ɹܣ��������� ��
��3���������ŷŵ�β���к���������SO ����ֹ��Ⱦ�������������ԭ�ϣ����ŷ�ǰ�������β���������跨�����ۺ����á�
����ֹ��Ⱦ�������������ԭ�ϣ����ŷ�ǰ�������β���������跨�����ۺ����á�
��ͳ�ķ����ǣ�β���е�SO ͨ����������ˮ���գ�Ȼ������ϡ���ᴦ����д�����������еĻ�ѧ��Ӧ����ʽ��
�����ŵ���
��
ͨ����������ˮ���գ�Ȼ������ϡ���ᴦ����д�����������еĻ�ѧ��Ӧ����ʽ��
�����ŵ���
��
���·����ǣ���β���е�SO ��Na
��Na SO
SO ��Һ���գ�Ȼ���ټ���������Һ��д�����������еĻ�ѧ��Ӧ����ʽ��
�����·����봫ͳ������ȣ����ŵ��� ��
��Һ���գ�Ȼ���ټ���������Һ��д�����������еĻ�ѧ��Ӧ����ʽ��
�����·����봫ͳ������ȣ����ŵ��� ��
��4��������Ĺ�ҵ�Ʒ��У���������������˵��������������Ҫԭ����߶���ȷ���� ������ţ�
A���ӷ���¯������¯���辻������Ϊ¯���е�SO �����ʷ�Ӧ
�����ʷ�Ӧ
B�����������г����ø�ѹ������Ŀ�������SO ��ת����
��ת����
C��SO ������ΪSO
������ΪSO ʱ��Ҫʹ�ô����������������SO
ʱ��Ҫʹ�ô����������������SO ��ת����
��ת����
D��SO ��98.3%Ũ�������գ�Ŀ���Ƿ�ֹ�γ�������������SO
��98.3%Ũ�������գ�Ŀ���Ƿ�ֹ�γ�������������SO ������ȫ
������ȫ
��5��ij���᳧��Ҫ����8��98%��Ũ����������Ҫ��״���µĿ���
m ( O
( O ��������������20%����)��
��������������20%����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com