

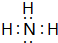 ����
����
 ����
�������� ��ͼ�еĻ��ϼۺ�ԭ�Ӱ뾶�Ĵ�С����֪x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�أ�
��1��f��AlԪ�أ���Ԫ�����ڱ���λ���ǵ������ڢ�A�壻
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС���ǽ�����Խǿ�����������ˮ���������Խǿ��
��3����ԭ�ӹ��ۻ����������NH3��H2O2��C2H2�ȣ�
��4��1molNa�ĵ���������O2��ȼ������Na2O2��s�����ų�255.5kJ������2molNa��Ӧ�ų�����Ϊ511kJ��ע���ۼ�״̬����Ӧ����д�Ȼ�ѧ����ʽ��
��5����R��NH4Al��SO4��2����Һ��Al3+��NH4+��ˮ��ʹ��Һ�����ԣ���Al3+�� NH4+ˮ��̶ȸ���
��m������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3•H2O��
�۸���n=cV����n��Al3+ ����n��NH4+����n��SO42-����n��Ba2+����n��OH-��������SO42-��Ba2+�в����������ӵ����ʵ�����������BaSO4�����ʵ��������η�����Al3++OH-=Al��OH��3����NH4++OH-=NH3•H2O��Al��OH��3+OH-=AlO2-+2H2O�����ݷ���ʽ��������Al��OH��3�����ʵ������������������ɹ��������ʵ�����
��� �⣺��ͼ�еĻ��ϼۺ�ԭ�Ӱ뾶�Ĵ�С����֪x��HԪ�أ�y��CԪ�أ�z��NԪ�أ�d��OԪ�أ�e��NaԪ�أ�f��AlԪ�أ�g��SԪ�أ�h��ClԪ�أ�
��1��f��AlԪ�أ���Ԫ�����ڱ���λ���ǵ������ڢ�A�壬�ʴ�Ϊ���������ڢ�A�壻
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��r��O2-����r��Na+�����ǽ�����Խǿ�����������ˮ���������Խǿ�������ԣ�HClO4��H2SO4���ʴ�Ϊ��r��O2-����r��Na+����HClO4��H2SO4��
��3����ԭ�ӹ��ۻ����������NH3��H2O2��C2H2�ȣ������ʽΪ��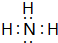 ����
����
 ����
����
�ʴ�Ϊ��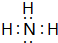 ����
����
 ����
����
��4��1molNa�ĵ���������O2��ȼ������Na2O2��s�����ų�255.5kJ������2molNa��Ӧ�ų�����Ϊ511kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ��2Na��s��+O2��g��=Na2O2��s����H=-511kJ•mol-1��
�ʴ�Ϊ��2Na��s��+O2��g��=Na2O2��s����H=-511kJ•mol-1��
��5����R��NH4Al��SO4��2����Һ��Al3+��NH4+��ˮ��ʹ��Һ�����ԣ���Al3+�� NH4+ˮ��̶ȸ�������Ũ���ɴ�С��˳���ǣ�c��SO42-����c��NH4+����c��Al3+����c��H+����c��OH-����
�ʴ�Ϊ��c��SO42-����c��NH4+����c��Al3+����c��H+����c��OH-����
��m������м����������ƣ��������ʵ������䣬��NH4+��OH-��Ӧ����NH3•H2O�����ӷ���ʽΪ��NH4++OH-=NH3•H2O��
�ʴ�Ϊ��NH4++OH-=NH3•H2O��
��10mL 1mol•L-1 NH4Al��SO4��2��Һ��Al3+ ���ʵ���Ϊ0.01mol��NH4+�����ʵ���Ϊ0.01mol��SO42-�����ʵ���Ϊ0.02mol��20mL 1.2 mol•L-1Ba��OH��2��Һ��Ba2+���ʵ���Ϊ0.024mol��OH-Ϊ0.048mol��
��SO42-+Ba2+=BaSO4������֪SO42-���㣬�ʿ��Եõ�0.02mol BaSO4��
Al3++3OH-=Al��OH��3��
0.01mol 0.03mol 0.01mol
��Ӧʣ��OH-Ϊ0.048mol-0.03mol=0.018mol��
NH4++OH-=NH3•H2O
0.01mol 0.01mol
��Ӧʣ��OH-Ϊ0.018mol-0.01mol=0.008mol��
Al��OH��3+OH-=AlO2-+2H2O
0.008mol 0.008mol
�ʵõ�Al��OH��3����Ϊ0.01mol-0.008mol=0.002mol
�����յõ�����Ϊ0.02mol+0.002mol=0.022mol��
�ʴ�Ϊ��0.022mol��
���� ���⿼��ṹλ�����ʹ�ϵ�����Ӱ뾶�Ĵ�С�Ƚϡ�Ԫ�������ɡ��Ȼ�ѧ����ʽ��д������Ũ�ȴ�С�Ƚϡ���ѧͼ��ѧ���㣬�Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
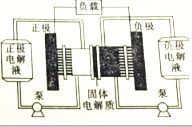 ȼ�ϵ��ʵ���ϲ��ܡ����硱����һ�����糧��ij�-ͭ����ȼ�ϵ��ͨ��һ�ָ��ӵġ�ͭ��ʴ��������·�����зŵ����Ϊ2Li+Cu2O+H2O�T2Cu+2Li++2OH-������˵����ȷ���ǣ�������
ȼ�ϵ��ʵ���ϲ��ܡ����硱����һ�����糧��ij�-ͭ����ȼ�ϵ��ͨ��һ�ָ��ӵġ�ͭ��ʴ��������·�����зŵ����Ϊ2Li+Cu2O+H2O�T2Cu+2Li++2OH-������˵����ȷ���ǣ�������| A�� | �õ��Ӧ���ڸ���ͨ����� | |
| B�� | �ŵ�ʱ��Li+�������������ƶ� | |
| C�� | �ŵ�ʱ�������ĵ缫��ӦʽΪCu2O+2e-�T2Cu+O2- | |
| D�� | ������Ӧ�����У�ͭ�൱�ڴ�����������ʵ����O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
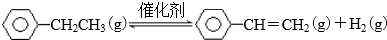
| ��ѧ�� | C-H | C-C | C=C | H-H |
| ����/kJ•mol��1 | 412 | 348 | 612 | 436 |
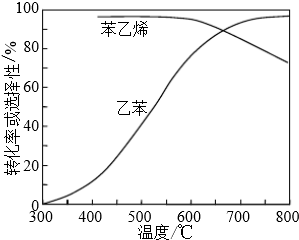
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȼ�������ȼ������SO3 | |
| B�� | �й��Ŵ�����������Һ���������ͭ�������ͭ�� | |
| C�� | ���ð�˾ƥ�ֳ���ˮ���ᷴӦʱ����NaHCO3��Һ�ⶾ | |
| D�� | ʹ�ú�������Ũ�Ƚϴ�ĵ���ˮϴ�·�������ȥ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ17��������Ϊ20����ԭ��${\;}_{17}^{20}$Cl | |
| B�� | �����ӣ�Cl-���Ľṹʾ��ͼ��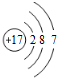 | |
| C�� | �ȷ��ӵĵ���ʽ��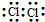 | |
| D�� | ����ϩ���ӵĽṹ��ʽ��H3C-CH2Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ��Cl2+H2O=2H++Cl-+ClO- | |
| B�� | Na2CO3��Һ��CO32-��ˮ�⣺CO32-+H2O=HCO3-+OH- | |
| C�� | ������Һ��KIO3��KI��Ӧ����I2��IO3-+I-+6H+=I2+3H2O | |
| D�� | NaHCO3��Һ�м�����Ba��OH��2��Һ��HCO3-+Ba2++OH-=BaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| t/s | 0 | t1 | t2 | t3 | t4 |
| n��so3��/mol | 0 | 0.6 | 1.2 | 1.8 | 1.8 |
| A�� | ��Ӧ��ǰt1s��ƽ������v��O2��=0.3/t1 mol•L-1•s-1 | |
| B�� | ���¶��·�Ӧƽ�ⳣ��Ϊ1.62��10-3L/mol | |
| C�� | ��ͬ�¶��£���ʼʱ�������г���4 mol SO3���ﵽƽ��ʱ��SO3��ת���ʴ���10% | |
| D�� | �¶Ȳ��䣬����������ٳ���0.2 mol SO2��0.1 mol O2��1.8 mol SO3��Ӧ�ﵽ��ƽ��ʱSO3ת�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Һ�м���������NaOH��HCl��Һʱ����Һ��pH�п��ܲ��ᷢ�������仯 | |
| B�� | ��Һ����������֮���п������㣺c��Na+����c��CH3COO-����c��OH-����c��H+�� | |
| C�� | ����Һ������ΪCH3COONa��NaOHʱ����һ����c��Na+����c��OH-����c��CH3COO-����c��H+�� | |
| D�� | ����������֮��������c��CH3COO-����c��Na+����c��H+����c��OH��ʱ������Һ������һ����CH3COONa��CH3COOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com