
 2007��ŵ������ѧ������¹���ѧ�Ҹ����?���ض����Ա������ڱ��滯ѧ�о����������Ŀ����Թ��ף�
2007��ŵ������ѧ������¹���ѧ�Ҹ����?���ض����Ա������ڱ��滯ѧ�о����������Ŀ����Թ��ף�
| ||
| ||
| ||
| 1 |
| 3 |
| 1 |
| 3 |
| ||


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״̬�£�22.4Lˮ�к��е���ԭ����ΪNA |
| B�����³�ѹ�£�8gO2����4 NA������ |
| C��25��ʱ��pH=13��1L Ba��OH��2��Һ�к���OH-����ĿΪ0.2NA |
| D��53.5gNH4Cl�к���H-Cl���ĸ���Ϊ4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
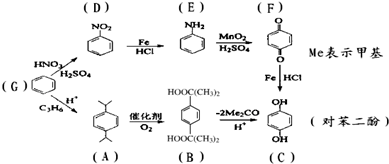
| A��G��D��D��E�ķ�Ӧ������ȡ����Ӧ |
| B��B�л����к��еĹ��������Ȼ� |
| C��G��A��A��B��������Ӧ����������ԭ�ӱ�100%���� |
| D���Ա����ӿ��Է���ȡ�����ӳɡ���ԭ�����������ۺ�ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
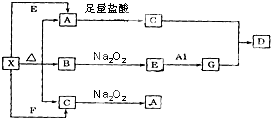 ��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪��ɫ�����ĩX���ȷֽ⣬�ָ����������ɰ�ɫ����A����ɫҺ��B����ɫ����C��X��E��G����ɫ��Ӧ��Ϊ��ɫ��DΪ��ɫ������
��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪��ɫ�����ĩX���ȷֽ⣬�ָ����������ɰ�ɫ����A����ɫҺ��B����ɫ����C��X��E��G����ɫ��Ӧ��Ϊ��ɫ��DΪ��ɫ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
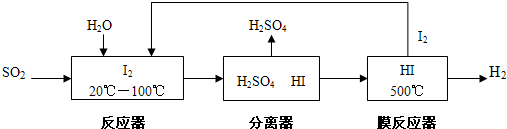
| ��Ӧʱ��/min | n��CO��/mol | H2O/mol |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com