
Ԫ�� | O | S | Se | Te |
������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
��1������Ԫ���γɵ��⻯����ȶ�����ǿ������˳����___________________________��
��2����ҵ��Al2Te3�������Ʊ�H2Te��������л�ѧ����ʽ����ƽ��
�� ��Al2Te3+�� ��____________====�� ��Al(OH)3��+�� ��H2Te��
��3����101 kPaʱ��
��1��H2O��H2S��H2Se��H2Te
��2��1 6H2O 2 3
��3��S(s)+O2(g)====SO2(g);��H=-296 kJ��mol-1
���������⿼����Ԫ�������ɵ�֪ʶ�ͻ�ѧ����ʽ���Ȼ�ѧ����ʽ����д�����⣨1������������Ԫ���⻯����ȶ��ԣ��ǽ���Ԫ�ص�������������ʱԽ���ף����ɵ��⻯����ȶ���Խǿ����֮���ȶ���Խ������������Ϣ�ɵó��ȶ���ǿ����H2O��H2S��H2Se��H2Te�����⣨2�������˻�ѧ����ʽ����д�����ݷ�Ӧǰ��Ԫ���غ����ȷ��δ֪��Ӧ��Ļ�ѧʽ�����ʵļ���������ѧ����ʽΪ��Al2Te3+6H2O====2Al(OH)3��+3H2Te�������⣨3���������Ȼ�ѧ����ʽ����д����д�Ȼ�ѧ����ʽʱҪע�������ʵ�״̬�����ȷ�Ӧ�Ħ�H��0�����������Ϣ���Լ����1 mol�����ȫȼ������SO2�ų�������Ϊ296 kJ����ȼ�յĻ�ѧ����ʽΪ��S(s)+O2(g)====SO2(g);��H=-296 kJ��mol-1��


 �������ϵ�д�
�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
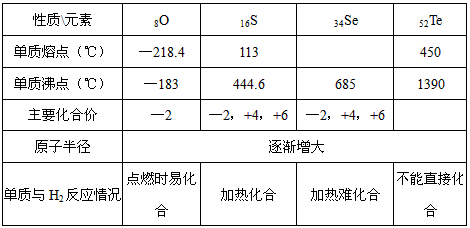
 ���������ǿ�ѧѧϰ����Ҫ����֮һ����ѧϰ������Ԫ�صĸ������ʺɹ������������±���ʾ�ı����֣���
���������ǿ�ѧѧϰ����Ҫ����֮һ����ѧϰ������Ԫ�صĸ������ʺɹ������������±���ʾ�ı����֣���| ����\Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | -218.4 | 113 | 217 | 450 |
| ���ʷе㣨�棩 | -183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | -2 | -2��+4��+6 | -2��+4��+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

����ݱ��ش��������⣺
(1)�����۵㷶Χ������_____________��
(2)�ڵĻ��ϼۿ�����_____________��
(3)�������ڵ��⻯��ˮ��Һ��������ǿ����˳����_____________(�ѧʽ)��
(4)�������н�ǿ��(������ԡ���ԭ�ԡ�)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ____________________________________��
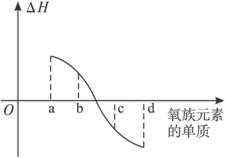
(5)��ͼ��ʾΪ����Ԫ�ص�����H2��Ӧ�����е������仯(��H)ʾ��ͼ������a��b��c��d�ֱ��ʾ������ijһԪ�صĵ��ʣ���HΪ��ͬ���ʵ����ĵ�����H2��Ӧ�ķ�Ӧ�ȡ���b����___________(д��������)��
(6)�����ۺ�����(H2SO4)��һ����;�㷺�Ļ�ѧ�Լ�����ҵ��������ʱͨ��Ҫ�����ٺ������ʵ����� ��SO2��������SO3 ��SO3������������Ҫ���̡���֪SO2�Ĵ�������һ�����ȵĿ��淴Ӧ���÷�Ӧ��ѧƽ�ⳣ��(K)�ı���ʽΪ_______________________��Ϊ���SO2��ת���ʣ���д�����ֿ��Բ��õĴ�ʩ��_____________________________��_______________________________������SO3ʱ����Ӧ������γ����������Ƿ������ˮֱ�����գ�________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ�ص�����H2��Ӧ���Ϊ��
| Ԫ�� | O | S | Se | Te |
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
������Ԫ���γɵ��⻯����ȶ�����ǿ������˳���ǣ� ��
�ƹ�ҵ��Al2Te3�������Ʊ�H2Te��������л�ѧ����ʽ����ƽ��
( )A12Te3��( ) ��( )Al(OH)3����( )H2Te��
����101kPaʱ��4.0g�������������ȫȼ������SO2���ų�37kJ������������ȼ�յ�
�Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0120 ģ���� ���ͣ������
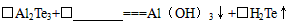

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com