
��֪��CH3COONa��NaOH ![]() Na2CO3��CH4��������ͼ��A��B��C��D��E��F��Ϊ�������壬A��B�����к�����ͬ��Ŀ�ĵ��ӣ�G��H�о�������Ԫ�أ�������Ӧ���ɵ�ˮ����ȥ����Ӧ����δע�������Իش�
Na2CO3��CH4��������ͼ��A��B��C��D��E��F��Ϊ�������壬A��B�����к�����ͬ��Ŀ�ĵ��ӣ�G��H�о�������Ԫ�أ�������Ӧ���ɵ�ˮ����ȥ����Ӧ����δע�������Իش�
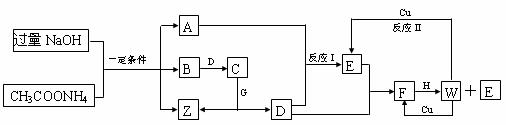
��д��A�ĵ���ʽ ��
��Z��Һ�и�����Ũ�ȴ�С��ϵΪ ��
����101kPaʱ��B��ȼ����ԼΪ890kJ/mol����Bȼ�յ��Ȼ�ѧ����ʽΪ ��
�ȷ�Ӧ������ӷ���ʽ ��
������������Ӧ�У����������ʼ���������������ԭ���������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����25�������½�pH=3�Ĵ���ϡ��100������Һ��pHΪ
��1����25�������½�pH=3�Ĵ���ϡ��100������Һ��pHΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
��CH3COONa+NaOH![]() CH4��+Na2CO3
CH4��+Na2CO3
��R��CH==CH2+H2O![]() RCH2CH2OH
RCH2CH2OH
���з���ʽΪC8H9Br���л�����һ���������ܷ�����ͼ��ʾ��һϵ�з�Ӧ��

������������⣺
(1)д��C8H9Br�Ľṹ��ʽ��__________��
(2)������Ӧ������ȡ����Ӧ����(����) __________����Ӧ�ۢܢߢ�����������ͷ�Ӧ__________________________________________________��
(3)д���ۢޢ�������Ӧ�Ļ�ѧ����ʽ��
��____________________________________________________________;
��____________________________________________________________;
��____________________________________________________________��
(4)д��B�����е�3��ͬ���칹��(Ҫ�ٱ�����ֻ��һ��ȡ��������3���칹�岻ͬ���)��
______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��7�֣�
��1����25�������½�pH=3�Ĵ���ϡ��100������Һ��pHΪ
A��5 B��1 C��1��3֮�� D��3��5֮��
��2�������£�pH=3�������pH=11�İ�ˮ�������Ϻ����Һ��
c��Cl���� c��NH4�������>������=����<������
��3�������£���֪ijCH3COONa��Һ�е�c��Na����=c��CH3COOһ���������Һ��pH 7���>������=����<������
��4�������NaOH��NH4Cl��Һ��Ũ�ȡ��������ϣ�������Һ�и�����Ũ���ɴ�С��˳��Ϊ��c( ) c( ) c( ) c( ) c( )
[�����������ӷ��ţ��� ������д��>����=��]
��5�������µ����ʵ���Ũ�ȵ�������Һ�У���NH4Al(SO4)2����NH4Cl����CH3COONH4����NH3��H2O��c(NH4+)�ɴ�С��˳���ǣ�_____________________________������ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ�������и���12���¿���ѧ�Ծ� ���ͣ������
��7�֣�
��1����25�������½�pH=3�Ĵ���ϡ��100������Һ��pHΪ
| A��5 | B��1 | C��1��3֮�� | D��3��5֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����12���¿���ѧ�Ծ� ���ͣ������
��7�֣�
��1����25�������½�pH=3�Ĵ���ϡ��100������Һ��pHΪ
A��5 B��1 C��1��3֮�� D��3��5֮��
��2�������£�pH=3�������pH=11�İ�ˮ�������Ϻ����Һ��
c��Cl���� c��NH4�������>������=����<������
��3�������£���֪ijCH3COONa��Һ�е�c��Na����=c��CH3COOһ���������Һ��pH 7���>������=����<������
��4�������NaOH��NH4Cl��Һ��Ũ�ȡ��������ϣ�������Һ�и�����Ũ���ɴ�С��˳��Ϊ��c( ) c( ) c( ) c( ) c( )
[�����������ӷ��ţ��� ������д��>����=��]
��5�������µ����ʵ���Ũ�ȵ�������Һ�У���NH4Al(SO4)2����NH4Cl����CH3COONH4����NH3��H2O��c(NH4+) �ɴ�С��˳���ǣ�_____________________________������ţ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com