
��ͼ�е�ÿһ�������е���ĸ��ʾ�йص�һ�ַ�Ӧ�����������е������ֿ��ڵ����ʱ�ʾ��ʼ��Ӧ���Ӧ�м����ˮ�����ɵ�ˮ�Լ����ɳ���Jʱ���������������ȥ����
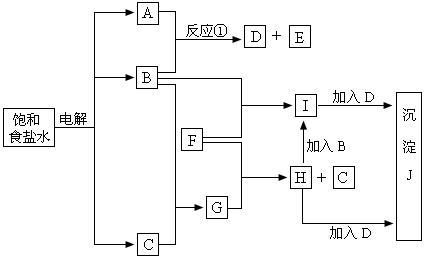
��������пհף�
��1������B��________��F��________��J��________��
��2����Ӧ�ٵ����ӷ���ʽ��________��
��3����H�м���B�����ӷ���ʽ��________��
��4����H�м���D������J�����������������Ļ�ѧ��Ӧ�Т�________��
��________����________��
|
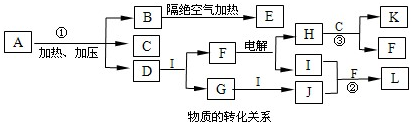
�����Ե�ⱥ��ʳ��ˮ��Ԫ�ػ���������ʣ����һ������ķ�Ӧ���磬�ѻ���������Ԫ�ػ�����֪ʶ���ϣ��ۺϿ���ѧ����˼ά������������������ ��������ʱ��Ҫ�ӵ�ⱥ��ʳ��ˮ����
�� �ٴӱ���ͼ��һ��ʼ��F��
��� ���1��B�� ��2����Ӧ��Ϊ ��3�� ��4���� ��
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


 NH4++NH2-
NH4++NH2- NH4++NH2-
NH4++NH2-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ƽһ�߸߶���һ���¿���ѧ�Ծ����������� ���ͣ������
��10�֣���ͼ��ÿһ�����е���ĸ����һ�ַ�Ӧ����������֪��A��B��C��D��Ϊǰ������Ԫ�صĵ��ʣ�Ҳ����ѧ��ѧ�̲��г��������ʡ�����A��BΪ���壬��B�����õİ뵼����ϣ�C��DΪ���壬���H�ı�����Һ����x��C��D���˷�Ӧ��һ����Ҫ�Ļ�����Ӧ(���ʼ��ת��ʱ�μӷ�Ӧ�����ɵ�H2O����ȥ)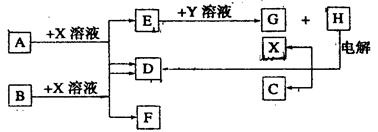
(1)B����Ϊ�����������������о�B���������һ����Ҫ�Ĺ�ҵ��;Ϊ������������������
(2)X�ĵ���ʽΪ������������Y��(�ѧʽ)������������
(3)E��G��ˮ��Һ�л�Ϸ�Ӧ�������ӷ�Ӧ����ʽΪ��������������������
������������������������������������������
����E��ˮ��Һ��ͨ�������CO2�������ӷ�Ӧ����ʽΪ����������������
������������������������������������������
(4)25��ʱ��ʯī�缫��⺬0��2 mol H��ˮ��Һ������������0��2 mol����ʱ����ô�ʱ��Һ�����Ϊ200 mL�������Һ��C(OH��)��ӽ�����ֵΪ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ��ƽһ�߸߶���һ���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣���ͼ��ÿһ�����е���ĸ����һ�ַ�Ӧ����������֪��A��B��C��D��Ϊǰ������Ԫ�صĵ��ʣ�Ҳ����ѧ��ѧ�̲��г��������ʡ�����A��BΪ���壬��B�����õİ뵼����ϣ�C��DΪ���壬���H�ı�����Һ����x��C��D���˷�Ӧ��һ����Ҫ�Ļ�����Ӧ(���ʼ��ת��ʱ�μӷ�Ӧ�����ɵ�H2O����ȥ)
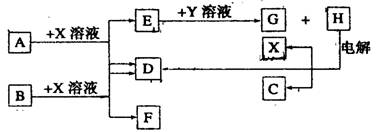
(1)B����Ϊ�����������������о�B���������һ����Ҫ�Ĺ�ҵ��;Ϊ������������������
(2)X�ĵ���ʽΪ������������Y��(�ѧʽ)������������
(3)E��G��ˮ��Һ�л�Ϸ�Ӧ�������ӷ�Ӧ����ʽΪ��������������������
������������������������������������������
����E��ˮ��Һ��ͨ�������CO2�������ӷ�Ӧ����ʽΪ����������������
������������������������������������������
(4)25��ʱ��ʯī�缫��⺬0��2 mol H��ˮ��Һ������������0��2 mol����ʱ����ô�ʱ��Һ�����Ϊ200 mL�������Һ��C(OH��)��ӽ�����ֵΪ������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com