
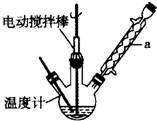
 �ױ���
�ױ���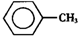 ����һ����Ҫ�Ļ���ԭ�ϣ���������������ȩ��
����һ����Ҫ�Ļ���ԭ�ϣ���������������ȩ�� ���������ᣨ
���������ᣨ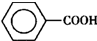 ���Ȳ�Ʒ���±��г����й����ʵIJ����������ʣ���ش�
���Ȳ�Ʒ���±��г����й����ʵIJ����������ʣ���ش�| �� | ��״ | �۵㣨�棩 | �е㣨�棩 | ����ܶȣ���ˮ=1g/cm3�� | �ܽ��� | |
| ˮ | �Ҵ� | |||||
| �ױ� | ��ɫҺ����ȼ�ӷ� | -95 | 110.6 | 0.8660 | ���� | ���� |
| ����ȩ | ��ɫҺ�� | -26 | 179 | 1.0440 | �� | ���� |
| ������ | ��ɫƬ״����״���� | 122.1 | 249 | 1.2659 | �� | ���� |
 ���˷�Ӧ��ԭ�������������Ͽɴ�66.25%��
���˷�Ӧ��ԭ�������������Ͽɴ�66.25%������ ��1��������a�ṹ��֪Ϊ���������ܣ��ױ��ӷ��ᵼ�²��ʽ��ͣ�����ƿ�мױ������������������ɱ���ȩ��ͬʱ������ˮ��ԭ��������=��Ԥ�ڲ������������ȫ����Ӧ�������������100%��
��2���¶ȹ���ʱ��������ֽ⣬ʵ�ʲμӷ�Ӧ�Ĺ�������������С��Ӱ�������
��3����Ӧ��Ϻ�Ӧ���Һ������Ȼ��ȴ������ʱ���ȹ��˷���������������������ķ�������������
��4��ʵ���м�������Ĺ������Ⲣ�ӳ���Ӧʱ��ʱ����ʹ����ȩ��Ʒ�в����϶�ı����ᣮ
������̼�����Ʒ�Ӧ֮��Ϊ�������ƣ��ٷ�Һ���룬ˮ���м�������õ������ᾧ�壬�����˷���õ������
�ڱ���������ʵ�����������KOH���ʵ������ټ����Ʒ�б����������������
��a���ζ�ʱ���Ӷ�ȡ�ļ�������ʹKOH��Һ�������ƫС��
b��KOH��Һ��ʱ��Ӵ������������ն�����̼����ת��Ϊ̼��أ�������������������Һ���ƫ��
c������KOH��Һʱ���Ӷ��ݣ�Һ���ڿ̶����Ϸ������Ʊ�Һ�����ƫ��Ũ��ƫС�����µζ�ʱ����KOH��Һ���ƫ��
d������ָ̪ʾ����Ϊ������Һ���յ�ʱ��Һ�����ԣ���������������Һ���ƫС��
��� �⣺��1������a�������������������ܣ�����ΪΪ���������ܣ��ױ��ӷ��ᵼ�²��ʽ��ͣ�������������ֹ�ױ��ӷ����²��ʽ��ͣ�����ƿ�мױ������������������ɱ���ȩ��ͬʱ������ˮ����Ӧ����ʽΪ�� ���˷�Ӧ��ԭ��������=$\frac{106}{160}$��100%=66.25%��
���˷�Ӧ��ԭ��������=$\frac{106}{160}$��100%=66.25%��
�ʴ�Ϊ�����������ܣ�������������ֹ�ױ��Ļӷ������Ͳ�Ʒ���ʣ� ��66.25%��
��66.25%��
��2�����¶ȹ���ʱ��������ֽ��ٶȼӿ죬ʵ�ʲμӷ�Ӧ�Ĺ�������������С������ȩ�IJ���ȴ�������٣�
�ʴ�Ϊ���¶ȹ���ʱ��������ֽ��ٶȼӿ죬ʵ�ʲμӷ�Ӧ�Ĺ�������������С��Ӱ�������
��3����Ӧ��Ϻ�Ӧ���Һ������Ȼ��ȴ������ʱ���ȹ��˷���������������������ķ�������������ᣬ
�ʴ�Ϊ�����ˡ�����
��4��ʵ���м�������Ĺ������Ⲣ�ӳ���Ӧʱ��ʱ����ʹ����ȩ��Ʒ�в����϶�ı����ᣮ
������̼�����Ʒ�Ӧ֮��Ϊ�������ƣ��ٷ�Һ���룬ˮ���м�������õ������ᾧ�壬�����˷���õ������ᣬ��ϴ�ӡ�����õ������ᣬ����ȷ�IJ��������ǣ�dacb��
�ʴ�Ϊ��dacb��
������ʵ����л�õı������Ʒ���д��Ȳⶨ���ɳ�ȡ1.2g��Ʒ������200mL�Ҵ������Һ����ȡ���õ��Ҵ���Һ20.00mL����ƿ���μ�2��3�η�ָ̪ʾ����Ȼ����Ԥ����õ�0.1000mo/L KOH��Һ�ζ�������ζ��յ�ʱ����KOH��Һ18.00mL��
����������ʵ�����������KOH���ʵ�������2.500g��Ʒ�б�����Ϊ0.018L��0.1mol/L��$\frac{100}{20}$��122g/mol=1.098g����Ʒ�б��������������Ϊ$\frac{1.098g}{1.2g}$��100%=91.50%��
�ʴ�Ϊ��91.50%��
��a���ζ�ʱ���Ӷ�ȡ�ļ�������ʹKOH��Һ�������ƫС�����㱽���������ƫС���ⶨ���ƫ�ͣ���a���ϣ�
b��KOH��Һ��ʱ��Ӵ������������ն�����̼����ת��Ϊ̼��أ�������������������Һ���ƫ�ⶨ���ƫ�ߣ���b�����ϣ�
c������KOH��Һʱ���Ӷ��ݣ�Һ���ڿ̶����Ϸ������Ʊ�Һ�����ƫ��Ũ��ƫС�����µζ�ʱ����KOH��Һ���ƫ�ⶨ���ƫ�ߣ���c�����ϣ�
d������ָ̪ʾ����Ϊ������Һ���յ�ʱ��Һ�����ԣ���������������Һ���ƫС���ⶨ���ƫ�ͣ���d���ϣ�
�ʴ�Ϊ��ad��
���� ���⿼���л���ϳ�ʵ�顢���ʵķ����ᴿ��ʵ�鷽����ơ��Բ����ķ������ۡ����ʺ����ⶨ�ȣ��ϺõĿ���ѧ�������ݵ�Ӧ�á��Ķ���ȡ��Ϣ�������Լ�֪ʶǨ��Ӧ�ã��Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ����̿ | NO | N2 | X | |
| ��ʼ | 2.030 | 0.100 | 0 | 0 |
| T1 | 2.000 | 0.040 | 0.030 | 0.030 |
| T2 | 2.005 | 0.050 | 0.025 | 0.025 |
| A�� | ����X�Ļ�ѧʽΪCO2 | |
| B�� | T1��ʱ��ƽ�ⳣ��K1=$\frac{9}{32}$ | |
| C�� | ����������Ϣ�жϣ�T1��T2 | |
| D�� | T1��ʱ����ƽ����ϵ�м����������̿�������NO��ת���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
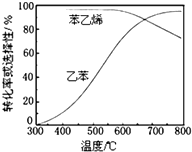 �ұ��������Ʊ���ϩ��Ӧ��
�ұ��������Ʊ���ϩ��Ӧ��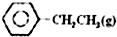 $\stackrel{����}{?}$
$\stackrel{����}{?}$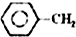 =CH2��g��+H2��g��
=CH2��g��+H2��g��| ��ѧ�� | C-H | C-C | C=C | H-H |
| ����/kJ•mol��1 | 412 | 348 | 612 | 436 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c1��c2=1��3 | |
| B�� | ��4v��X����=v��Y����ʱ���÷�Ӧ�������淴Ӧ������ƽ�� | |
| C�� | X��Y��ת���ʲ���� | |
| D�� | Y��ʼŨ��c2����Ϊ0.36mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
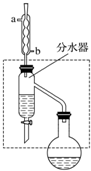 ���ᶡ������Ҫ�Ļ���ԭ�ϣ�����ˮ����ζ��ʵ�����Ʊ����ᶡ���ķ�Ӧ��װ��ʾ��ͼ���й���Ϣ���£�CH3COOH+CH3CH2CH2CH2OH$?_{��}^{ŨH_{2}SO_{4}}$ CH3COOCH2CH2CH2CH3+H2O
���ᶡ������Ҫ�Ļ���ԭ�ϣ�����ˮ����ζ��ʵ�����Ʊ����ᶡ���ķ�Ӧ��װ��ʾ��ͼ���й���Ϣ���£�CH3COOH+CH3CH2CH2CH2OH$?_{��}^{ŨH_{2}SO_{4}}$ CH3COOCH2CH2CH2CH3+H2O| ���� | ������ | ���ᶡ�� | |
| �۵�/�� | 16.6 | -89.5 | -73.5 |
| �е�/�� | 117.9 | 117 | 126.0 |
| �ܶ�/g•cm-3 | 1.1 | 0.80 | 0.88 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
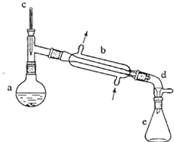 ������ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ�����£�
������ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ�����£�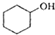 $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$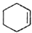 +H2O
+H2O| ��Է������� | �ܶ�/��g•cm3�� | �е�/�� | �ܽ��� | |
| ������ | 100 | 0.9618 | 161 | ����ˮ |
| ����ϩ | 82 | 0.8102 | 83 | ������ˮ |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������䣬�������ϵ��ѹǿ��ƽ�������ƶ�����CΪ���� | |
| B�� | �����������䣮�������¶ȣ�B��ƽ��ת����������÷�Ӧ�Ƿ��ȷ�Ӧ | |
| C�� | �÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{{c}^{4}��C��•{c}^{2}��D��}{{c}^{3}��A��•{c}^{2}��B��}$ | |
| D�� | ��ʱ��B��ƽ��ת������40% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com