
A��G����ѧ��ѧ�������ʣ�A��DΪ���ʣ�G�Ǻ�AԪ�ص��������塣��֪��
A(s)+B(g)====C(g)+D(g) ��H=+131.4 kJ��mol-1��
ijͬ ѧʵ���֪��4 g A����������Ӧ����43.8 kJ��������
ѧʵ���֪��4 g A����������Ӧ����43.8 kJ��������
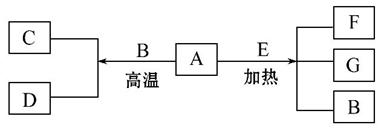
��1�� д��AԪ�ص�����________��
д��AԪ�ص�����________��
��2������֪��A(s)+O2(g)====G(g) ��H=-393.6 kJ��mol-1
C(g)+ O2(g)====G(g)����H=-283 kJ��mol-1
O2(g)====G(g)����H=-283 kJ��mol-1
D(g)+ O2(g)====B(g)����H=-242 kJ��mol-1
O2(g)====B(g)����H=-242 kJ��mol-1
�ɴ��жϡ���Ϊ283 kJ��mol-1+242 kJ��mol-1>393.6 kJ��mol-1������Aȼ��ʱ������B���Էų����������������˵���Ƿ���ȷ��___________��������___[.Com ]
]
____________________________________________________��
��3��д��A+O2��C���Ȼ�ѧ����ʽ��_______________________________��
.����������1����A(s)+B(g)====C(g)+D(g) ��H=+131.4 kJ��mol-1��
֪n(A)=43.8 kJ/131.4 kJ��mol-1��0.33 mol��
M(A)=4 g/0.33 mol��12 g��mol-1����AΪ̼��BΪH2O��CΪCO��DΪH2��GΪCO2��EΪŨ�����Ũ���ᡣ
��2���ɰ�3���Ȼ�ѧ����ʽ����дΪ��
C(s)+O2( g)====CO2(g)����H=-393.6 kJ��mol-1
g)====CO2(g)����H=-393.6 kJ��mol-1
CO(g)+ O2(g)====CO2(g) ��H=-283 kJ��mol-1
O2(g)====CO2(g) ��H=-283 kJ��mol-1
H2(g)+ O2(g)====H2O(g) ��H=-242 kJ��mol-1
O2(g)====H2O(g) ��H=-242 kJ��mol-1
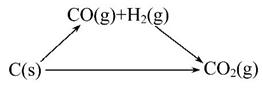
����;����Ӧ�ȷֱ�Ϊ��-283 kJ��mol-1-242 kJ��mol-1+131.4 kJ��mol-1=
-393.6 kJ��mol-1��-393.6 kJ��mol-1���ɼ�����ȵġ�
��3�� ��C(s)+O2(g)====CO2(g) ��H=-393.6 kJ��mol-1��
��C(s)+O2(g)====CO2(g) ��H=-393.6 kJ��mol-1��
CO(g)+ O2(g)====CO2(g)����H=-283 kJ��mol-1��ʽ�������C+
O2(g)====CO2(g)����H=-283 kJ��mol-1��ʽ�������C+ O2====CO���Ȼ�ѧ����ʽ��
O2====CO���Ȼ�ѧ����ʽ��
�𰸣���1��̼
��2������ȷ��1 mol A��O2ֱ��ȼ�շų�������Ϊ393.6 kJ����1 mol A����B��Ӧ����C��D��C��D����O2��Ӧ����������-131.4 kJ+283 kJ+242 kJ=
393.6 kJ��������ͬ
��3��C(s)+1/2O2(g)====CO(g) ��H=-110.6 kJ��mol-1


 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��Ȳ�뱽������ȫȼ�յ��Ȼ�ѧ����ʽ���£�
��C2H2(g)��5/2O2(g)����2CO2(g)��H2O(l)����H����1 300 kJ/mol
��C6H6(g)��15/2O2(g)����6CO2(g)��3H2O(l)����H����3 295 kJ/mol
����˵����ȷ����(����)
A��1 mol C2H2(g)��ȫȼ��������̬ˮʱ���ȴ���1 300 kJ
B��1 mol C6H6(l)��ȫȼ������Һ̬ˮʱ���ȴ���3 295 kJ
C����ͬ�����£���������C2H2(g)��C6H6(g)��ȫȼ�գ�C6H6(g)���ȸ���
D��C2H2(g)��������C6H6(g)�Ĺ������ڷ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧŪ�����Ȫʵ���ԭ��������һ�����µ��뷨������Ļ�һ����ƿ�ڵ����壬�Լ���ͷ�ιܺ��ձ��ڵ�Һ�壬ҲӦ�ÿ�������Ȫʵ�顣���ÿα������õ�װ��ʵ������һ�£�����õ�����������Ȫ����������в������Ǹ�ͬѧ��Ƶ���ϵ��ǣ� ��
A���Ȼ����ˮ B.������ˮ C.������̼��NaOH��Һ D.������NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A����ͼ��ʾ�Ĺ���ת��Ϊ������D��DΪǿ�ᣬ��ش��������⣺
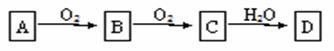
��1����A�ڳ�����Ϊ���嵥����ش�
��A��C�Ļ�ѧʽ�ֱ��ǣ�A________��C________��
�ڽ�Cͨ��ˮ��Һ�У���Ӧ��ѧ����ʽΪ_____________________________________________��
��2������A��B�ڳ�����Ϊ������Ϊ����� ��ش�
��A�Ļ�ѧʽ�ǣ�A_____________��
��B����C�Ļ�ѧ����ʽΪ________________________________________________________��
��һ��������̼������D��Ӧ�Ļ�ѧ����ʽΪ________________________________________���÷�Ӧ��D��������__________�ԡ�
��3����A�ڳ�����Ϊ���嵥����ش�
��D�Ļ�ѧʽ��___________��
����2mol D��Ũ��Һ�м���������Cu���ȣ���״���²������������_______22.4L������ڡ������ڡ���С�ڡ�����ԭ��Ϊ_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��˹������Ϊ���������غ�ģ����ܻ�ѧ��Ӧ������һ����ɻ�ּ�����ɣ��������̵���ЧӦ����ͬ�ġ���֪��
��H2O(g)====H2O(l)����H1=-Q1kJ��mol-1
��C2H5OH(g)====C2H5OH(l)����H2=-Q2kJ��mol-1
��C2H5OH(g)+3O2(g)====2CO2(g)+3H2O(g) ��H3=- Q3kJ��mol-1
��ʹ23 gҺ̬��ˮ�ƾ���ȫȼ�գ����ָ������£���ų�������Ϊ(��λ��kJ)�� ��
A.Q1+ Q2+ Q3���������� B.1.5Q1-0.5Q2+0.5Q3
C.0.5Q1-1.5Q2+0.5Q3 D.0.5(Q1+Q2+Q3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
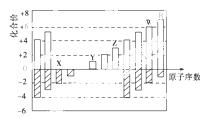
��ͼ��ʾԪ�����ڱ�ǰ�����ڵ�һ���֣�����Ԫ��X��Y��Z��W��������ȷ����(����)
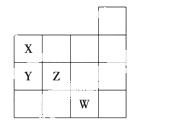
��X��Y������������Ӧ��ˮ���������Y��X
��Y��Z����̬�⻯����ȶ���Y��Z
��W�ĵ��ʳ����³�Һ̬���������۷�Ӧ
��W��ԭ��������Z��9
A��ֻ�Тۡ��������� B���٢�
C���٢ڢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
4���������������Ԫ�ص����λ�������Ԫ��X��ԭ�Ӻ����������M��2����Y��������������ԡ��ش��������⣺
| M | N | ||
| X | Y |
(1)Ԫ��X�����ڱ��е�λ���ǵ�________���ڡ���________�壬�䵥�ʿɲ��õ������________�ķ����Ʊ���
(2)M��N��Y����Ԫ������������Ӧ��ˮ�����У�������ǿ����________��������ǿ����________��(�ѧʽ)
(3)�������(MN)2�ĵ���ʽΪ________��(MN)2��Ϊ��±�أ�������±�����ƣ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ������˵����ȷ����(����)
A��ԭ�Ӱ뾶��Z>Y>X
B����̬�⻯����ȶ���R��W
C��WX3��ˮ��Ӧ�γɵĻ����������ӻ�����
D��Y��Z��������������Ӧ��ˮ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʯ[��Ҫ�ɷ�Ca5(PO4)3F]�ڸ������Ʊ�����(P4)���Ȼ�ѧ����ʽΪ��4Ca5(PO4)3F(s)��21SiO2(s)��30C(s)===3P4(g)��20CaSiO3(s)��30CO(g)��SiF4(g)����H
��������Ӧ�У����������������________��
����֪��ͬ�����£�
4Ca5(PO4)3F(s)��3SiO2(s)===6Ca3(PO4)2(s)��2CaSiO3(s)��SiF4(g)����H1
2Ca3(PO4)2(s)��10C(s)===P4(g)��6CaO(s)��10CO(g)����H2
SiO2(s)��CaO(s)===CaSiO3(s)����H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��____________��
(2)(����)��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
��Cu(s)��2H��(aq)===Cu2��(aq)��H2(g)
��H1����64.39 kJ·mol��1
��2H2O2(l)===2H2O(l)��O2(g)
��H2����196.46 kJ·mol��1
��H2(g)��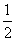 O2(g)===H2O(l)
O2(g)===H2O(l)
��H3����285.84 kJ·mol��1
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ_______________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com