
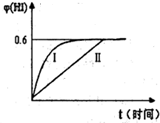
��9�֣���1��ijһ��Ӧ��ϵ�е������У�HCl��SnCl2��H2SnCl6��As��H3AsO3��H2O����֪��HCl�Ƿ�Ӧ��֮һ��
��д����ƽ�ĸ÷�Ӧ�Ļ�ѧ����ʽ��______________________ ��_______________________
�ڱ�������Ԫ���� ��
��2���⻯��ͭ(CuH)��һ���������ʣ���CuSO4��Һ�͡���һ���ʡ���40��50��ʱ��Ӧ����������CuH���е������У����ȶ����ֽ⣻����������ȼ�գ���ϡ���ᷴӦ���������壻Cu�������������·����ķ�Ӧ�ǣ�2Cu��===Cu2����Cu.
����������Ϣ������Լ������յĻ�ѧ֪ʶ���ش��������⣺
��д��CuH��������ȼ�յĻ�ѧ��Ӧ����ʽ��___________________________________.
�������CuH�ܽ���������ϡ���������ɵ�����ֻ��NO����д��CuH�ܽ�������ϡ�����з�Ӧ�����ӷ���ʽ��__________________________________________________.
��3�� ��25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22��68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________________________________
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| H | + 4 |
| O | - 2 |
| H | + 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣���1��ijһ��Ӧ��ϵ�е������У�HCl��SnCl2��H2SnCl6��As��H3AsO3��H2O����֪��HCl�Ƿ�Ӧ��֮һ��
��д����ƽ�ĸ÷�Ӧ�Ļ�ѧ����ʽ��______________________ ��_______________________
�ڱ�������Ԫ���� ��
��2���⻯��ͭ(CuH)��һ���������ʣ���CuSO4��Һ�͡���һ���ʡ���40��50��ʱ��Ӧ����������CuH���е������У����ȶ����ֽ⣻����������ȼ�գ���ϡ���ᷴӦ���������壻Cu�������������·����ķ�Ӧ�ǣ�2Cu��===Cu2����Cu.
����������Ϣ������Լ������յĻ�ѧ֪ʶ���ش��������⣺
��д��CuH��������ȼ�յĻ�ѧ��Ӧ����ʽ��___________________________________.
�������CuH�ܽ���������ϡ���������ɵ�����ֻ��NO����д��CuH�ܽ�������ϡ�����з�Ӧ�����ӷ���ʽ��__________________________________________________.
��3�� ��25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22��68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
�÷�Ӧ�л�ԭ������______________________������Ӧ������ת����0��2��
�ӣ������������������________________________��
��2��������Cl2 ͨ�� FeBr2��Һ�У���Ӧ�����ӷ���ʽΪ��________________________;
��3����NH4HCO3��Һ�еμ�������NaOH��Һ����Ӧ�����ӷ���ʽΪ______________;
��4���۲����·�Ӧ���ܽ���ɣ�Ȼ������������⣺
Al��OH��3 ��H2O Al��OH��4- + H+ NH3+H2O
NH4+ + OH-
����֪B��OH��3��һԪ���ᣬд������뷽��ʽ_____________________________
��N2H4�Ƕ�Ԫ���д����ڶ������뷽��ʽ___________________ ____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и�����ѧ�ڵڶ����¿����ۻ�ѧ�� ���ͣ������
��1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
�÷�Ӧ�л�ԭ������______________________������Ӧ������ת����0��2 ��
��
�ӣ������������������________________________ ��
��
��2��������Cl2 ͨ�� FeBr2��Һ�У���Ӧ�����ӷ���ʽΪ��________________________;
��3����NH4HCO3��Һ�еμ�������NaOH��Һ����Ӧ�����ӷ���ʽΪ______________;
��4���۲����·�Ӧ���ܽ���ɣ�Ȼ������������⣺
Al��OH��3 ��H2O Al��OH��4- + H+ NH3+H2O
Al��OH��4- + H+ NH3+H2O NH4+
+ OH-
NH4+
+ OH-
����֪B��OH��3��һԪ���ᣬд������뷽��ʽ_____________________________
��N2H4�Ƕ�Ԫ���д����ڶ������뷽��ʽ___________________ ____
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com