

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
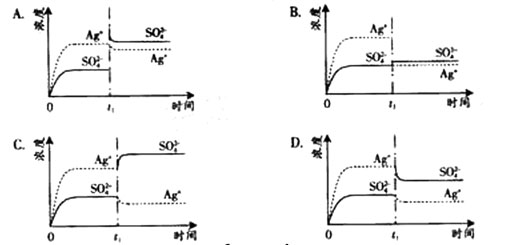
 )]2=KSP������KSPΪ��������Ϊ���¶���Mg(OH)2���ܶȻ����������ƶ�������þ�����������е��ܽ���ɴ�С��˳����
)]2=KSP������KSPΪ��������Ϊ���¶���Mg(OH)2���ܶȻ����������ƶ�������þ�����������е��ܽ���ɴ�С��˳����  AlCl3��Һ ��0.1mol��L
AlCl3��Һ ��0.1mol��L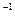 NH4C1��Һ
NH4C1��Һ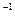 KCl��Һ ��0.1mol��L
KCl��Һ ��0.1mol��L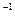 KAlO2��Һ
KAlO2��Һ| A���٢ܢۢ� | B���٢ڢۢ� | C���ۢ٢ܢ� | D���ܢ٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ksp��ӳ��������ˮ�е��ܽ���������������ֱ�Ӹ���Ksp����ֵ��С�Ƚϵ������ˮ�е��ܽ�������С |
| B���Ҵ������Ƕ������Ļ���� |
| C���ǽ���Ԫ�ص�ԭ�Ӽ�ֻ�γɹ��ۼ�������Ԫ�ص�ԭ����ǽ���Ԫ�ص�ԭ�Ӽ�ֻ�γ����Ӽ� |
| D���ҹ��涨�� 2008 �� 6 �� 1 �����̼Ҳ������ṩ���ϴ���Ŀ���ǽ��������ɱ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��NH4+��Mg2+��SO42����Cl�� | B��Ba2+��K+��OH����NO3- |
| C��Al3+��Cu2+ ��SO42����Cl�� | D��Na+��Ca2+��AlO2����Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na+��Cl-��Mg2+��K+ | B��Cu2+��SO42-��NO3-��K+ |
| C��NH4+��K+��CO32-��Cl- | D��Na+��K+��SO42-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ca2+��OH-��HCO3-��Cl- | B��Ba2+��Cu2+��SO42-��Cl- |
| C��Fe2+��Mg2+��SO42-��NO3- | D��Na+��Ag+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HCO3- | B��Cl- | C��OH- | D��Na+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com