
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ·mol��1��
2NH3(g)����H����92.4 kJ·mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
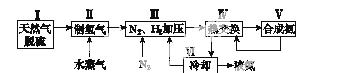
(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��______________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ·mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ·mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
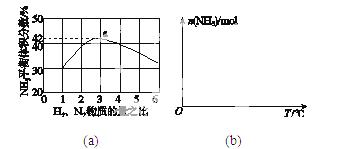
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����_______________________________________________________
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼���仯�����й㷺����;��
(1) ��ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ
C(s)��H2O(g) 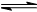 CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��
���Ϸ�Ӧ�ﵽƽ������������������£����´�ʩ���������H2O��ƽ��ת���ʵ���________��(�����)
A�������¶� B������̼������ C��������� D����CO���ռ���ȥCO
(2) ��֪��C(s)��CO2(g) 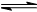 2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g)
2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g) 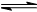 CO2(g)��H2(g)���ʱ䦤H��________��
CO2(g)��H2(g)���ʱ䦤H��________��
(3) CO��H2��һ�������¿ɷ�Ӧ���ɼ״���CO(g)��2H2(g) ===CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ_________________________________________________________________��
���øõ���ṩ�ĵ��ܵ��60 mL NaCl��Һ������0.01 mol CH3OH��ȫ�ŵ磬NaCl�������ҵ�������Cl2ȫ���ݳ������ǰ�������Һ����ı仯�����������������Һ��pH��________��
(4) ��һ������CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2.0 L�ĺ����ܱ������У��������·�Ӧ��
CO(g)��H2O(g)  CO2(g)��H2(g)���õ��������ݣ�
CO2(g)��H2(g)���õ��������ݣ�
| �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ���� ��ʱ��/min | ||
| H2O | CO | H2 | CO | ||
| 900 | 1.0 | 2.0 | 0.4 | 1.6 | 3.0 |
ͨ����������÷�Ӧ��ƽ�ⳣ��(���������λ��Ч����)________���ı䷴Ӧ��ijһ��������Ӧ���е�t minʱ����û��������CO2�����ʵ���Ϊ0.6 mol������200 mL 5 mol/L��NaOH��Һ������ȫ���գ���Ӧ�����ӷ���ʽΪ(��һ�����ӷ���ʽ��ʾ)________________________��
(5) ��ҵ�����ǰ�ˮú���еĻ�����徭���������õĽϴ�H2���ںϳɰ����ϳɰ���Ӧԭ��ΪN2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�����·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�����·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
��ش��������⣺����ʵ���Ƚϣ�ʵ���ı������Ϊ____________________��
��ʵ����ʵ�����¶�Ҫ�ߣ�����������ͬ������ͼ���л���ʵ����ʵ�����NH3Ũ����ʱ��仯��ʾ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��X��Y��Z������X��Zͬ���壬Y��Zͬ���ڣ�Zԭ��������������Xԭ���ڲ��������3������Yԭ��������������2��������˵���������
A��Yλ�ڵ�3���� B����X��Z���ֻһ��
C��ԭ�Ӱ뾶��Y > Z D����̬�⻯���ȶ��ԣ�Z > X
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�мס������������������ݻ��̶����������ݻ��ɱ䡣һ���¶���,�ڼ��м���2 mol N2��3 mol H2,��ӦN2(g)��3H2(g) 2NH3(g)�ﵽƽ��ʱ����NH3�����ʵ���Ϊm mol��
2NH3(g)�ﵽƽ��ʱ����NH3�����ʵ���Ϊm mol��
(1)��ͬ�¶��£������м���4 mol N2��6 mol H2�����ҵ�ѹǿʼ�����ѹǿ��ȣ����з�Ӧ�ﵽƽ��ʱ������NH3�����ʵ���Ϊ________ mol(�����и�����ѡ��ֻ����ţ���ͬ)�����ҵ��ݻ�����ݻ�ʼ����ȣ����з�Ӧ�ﵽƽ��ʱ������NH3�����ʵ���Ϊ________mol��
A����m���������������� B������ m
C����m��2m֮�� D������2m
E������2m
(2)��ͬ�¶��£������ҵ��ݻ�Ϊ��һ�룬������1 mol NH3��Ҫʹ���з�Ӧ�ﵽƽ��ʱ�������ʵ���������������������дﵽƽ��ʱ��ͬ������ʼʱӦ����________mol N2��________ mol H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10 L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g)  M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
| ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
| n(X) | n(Y) | n(M) | |||
| �� | 700 | 0.40 | 0.10 | 0.090 | |
| �� | 800 | 0.10 | 0.40 | 0.080 | |
| �� | 800 | 0.20 | 0.30 | a | |
| �� | 900 | 0.10 | 0.15 | b | |
����˵����ȷ����(����)
A��ʵ����У���5 minʱ���n(M)��0.050 mol����0��5 minʱ���ڣ���N��ʾ��ƽ����Ӧ����v(N)��1.0��10��2 mol·L��1·min��1
B��ʵ����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C��ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D��ʵ����У��ﵽƽ��ʱ��b>0.0 60
60
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڿ��淴Ӧ2SO2��O2 2SO3��ƽ��״̬�£����ֺ��º����������м���һ������O2������˵����ȷ����(KΪƽ�ⳣ����QcΪŨ����) (����)��
2SO3��ƽ��״̬�£����ֺ��º����������м���һ������O2������˵����ȷ����(KΪƽ�ⳣ����QcΪŨ����) (����)��
A��Qc���䣬K���O2ת��������
B��Qc���䣬K���SO2ת��������
C��Qc��С��K���䣬O2ת���ʼ�С
D��Qc����K���䣬SO2ת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ij��ѧ�о�С��̽����������Ի�ѧ��Ӧ���ʺͻ�ѧƽ��Ӱ���ͼ������ͼ���ʵ����۱������ȷ���� (����)��
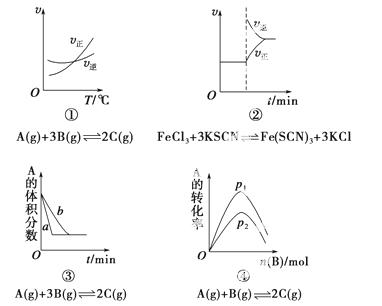
A��������������һ��ʱ����Ӧ�������¶ȱ仯��ͼ������Ӧ��H<0
B��������ƽ����ϵ����Һ����������KCl�����ѧ��Ӧ������ʱ��仯��ͼ��
C�������������������½�����ƽ�����ͼ��a��ʹ�ô���ʱ������
D������һ�������£�����һ����A������������Bʱ��ͼ��ѹǿp1>p2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�Ͽ���CO2�����״�����ӦΪCO2(g)��3H2(g)  CH3OH(g)��H2O(g)����6 mol CO2��8 mol H2����2 L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ7��2ʵ����ʾ��ͼ�����߱�ʾ���ı�ijһ��Ӧ����ʱ��H2���ʵ�����ʱ��ı仯������˵����ȷ����(����)
CH3OH(g)��H2O(g)����6 mol CO2��8 mol H2����2 L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ7��2ʵ����ʾ��ͼ�����߱�ʾ���ı�ijһ��Ӧ����ʱ��H2���ʵ�����ʱ��ı仯������˵����ȷ����(����)
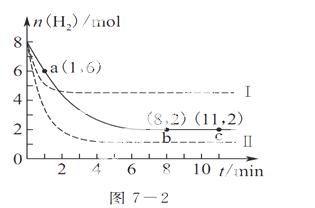
A�����ߢ��Ӧ�������ı��ǽ���ѹǿ
B�������ߢ��Ӧ�������ı��������¶ȣ���÷�Ӧ��H��0
C����Ӧ��ʼ��a��ʱv(H2)��1 mol·L��1·min��1
D�������������䣬�����÷�Ӧ���¶ȣ�ƽ�ⳣ��ֵ����С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʼ������Σ������������ε��ǣ� ��
A.NaHSO4 B.Na2SO4 C. Na2SO3 D.Na2S2O3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com