
£Ø14·Ö£©
¢ń£®²£Į§°ōŹĒ֊ѧ»ÆѧŹµŃéŹŅÖŠ³£ÓƵÄŅĒĘ÷”£ĻĀĮŠŹµŃé¹ż³ĢÖŠ£¬Ņ»°ć²»ŠčŅŖÓĆ²£Į§°ōµÄŹĒ
£ØĢīŠ“±ąŗÅ£©
¢ŁÓĆpHŹŌÖ½²ā¶ØNa2CO3ČÜŅŗµÄpH ¢ŚÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĀČ»ÆÄĘČÜŅŗ¢Ū½«ŹŹĮæĀČ»ÆĢś±„ŗĶČÜŅŗµĪČė·ŠĖ®ÖŠÖʱøĒāŃõ»ÆĢś½ŗĢå¢ÜĢ½¾æBa(OH)2![]() 8H20¾§ĢåŗĶNH4Cl¾§Ģå·“Ó¦¹ż³ĢÖŠµÄÄÜĮæ±ä»Æ ¢ŻÓĆÕōĮó·Ø·ÖĄėĮ½ÖÖ·Šµć²ī¾ą½Ļ“óµÄŅŗĢå ¢Ž¹żĀĖ·ÖĄė»„²»ĻąČܵĹĢĢåŗĶŅŗĢå¢ßÓĆŅŃÖŖÅØ¶ČµÄŃĪĖįµĪ¶Ø“ż²āÅØ¶ČµÄNaOHČÜŅŗµÄĖį¼īÖŠŗĶµĪ¶Ø¹ż³Ģ ¢ąĻ”ŹĶÅØH2SO4µÄ¹ż³Ģ
8H20¾§ĢåŗĶNH4Cl¾§Ģå·“Ó¦¹ż³ĢÖŠµÄÄÜĮæ±ä»Æ ¢ŻÓĆÕōĮó·Ø·ÖĄėĮ½ÖÖ·Šµć²ī¾ą½Ļ“óµÄŅŗĢå ¢Ž¹żĀĖ·ÖĄė»„²»ĻąČܵĹĢĢåŗĶŅŗĢå¢ßÓĆŅŃÖŖÅØ¶ČµÄŃĪĖįµĪ¶Ø“ż²āÅØ¶ČµÄNaOHČÜŅŗµÄĖį¼īÖŠŗĶµĪ¶Ø¹ż³Ģ ¢ąĻ”ŹĶÅØH2SO4µÄ¹ż³Ģ
¢ņ£®ĪŖ²ā¶Øijŗ¬ÓŠŌÓÖŹNa2OµÄNa2O2ѳʷµÄ“æ¶Č£¬Ä³Š”×éĶ¬Ń§·Ö±šÉč¼ĘĮĖČēĻĀ·½°ø”£
”¾·½°øŅ»”æ×¼Č·³ĘĮæѳʷmg£¬ÓėĖ®³ä·Ö·“Ó¦ŗó½«ČÜŅŗµÄĢå»żĻ”ŹĶĪŖVmL£¬“ÓÖŠČ”³öV1mLČÜŅŗ£¬×°Čė׶ŠĪĘ棬ÓĆŅŃÖŖÅØ¶ČµÄŃĪĖį½ųŠŠµĪ¶Ø£¬ŅŌČ·¶ØČÜŅŗµÄÅØ¶Č£¬ŌŁ¼ĘĖć³öѳʷ֊Na2O2µÄŗ¬Į攣
¢Å“Ė·½°øÖŠ£¬Ėį¼īÖŠŗĶµĪ¶ØŹ±Ó¦Ń”ÓĆ µĪ¶Ø¹Ü”£
¢ĘŠ“³ö“Ė·½°øÖŠÉę¼°µ½µÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
”¾·½°ø¶ž”æ×¼Č·³ĘĮæѳʷmg£¬½«ŃłĘ·Óė¶žŃõ»ÆĢ¼³ä·Ö·“Ó¦£¬Ķعż²ā¶Ø·“Ó¦²śÉśŃõĘųµÄĢå»ż£¬¼ĘĖć³öѳʷ֊Na2O2µÄŗ¬Į攣
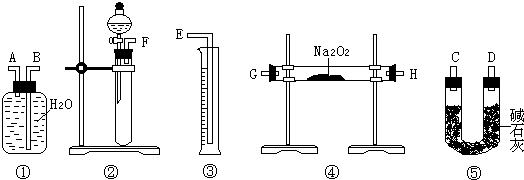
¢ĒøĆ·½°øµÄŹµŃé¹ż³ĢÖŠ£¬ŅĒĘ÷µÄĮ¬½ÓĖ³ŠņŹĒ £ØĢīŅĒĘ÷ĻĀ·½ŠņŗÅ£©£¬¢ŁÖŠµÄ½ųĘųæŚĪŖ £ØĢī”°A”±»ņ”°B”±£©
¢Č×°ÖĆ¢ŻµÄ×÷ÓĆŹĒ ”£
¢ÉÄćČĻĪŖ·½°øŅ»”¢·½°ø¶žÖŠ²ā¶Ø½į¹ū±Č½Ļ×¼Č·µÄŹĒ ”£
¢ń¢Ū¢Ż¢ß ¢ņ¢ÅĖįŹ½¢ĘNa2O+H2O=2Na++2OH- 2Na2O2+2H2O=4Na++4OH-+O2”ü H++OH-= H2O ¢Ē¢Ś¢Ü¢Ż¢Ł¢Ū A ¢Č³żČ„O2ÖŠ»ģÓŠµÄCO2µČĘųĢå ¢É·½°øŅ»
½āĪö:¢ń£ŗ¢ŁµÄ×÷ÓĆŹĒÕŗČ”“ż²āŅŗ£¬¢ŚµÄ×÷ÓĆŹĒ½Į°čŗĶŅżĮ÷£¬¢ÜµÄ×÷ÓĆŹĒ½Į°č£¬¢ŽµÄ×÷ÓĆŹĒŅżĮ÷£¬¢ąµÄ×÷ÓĆŹĒŅżĮ÷£»¢ņ¢ÅŹ¢ŃĪĖįµÄŅ»¶ØŹĒĖįŹ½µĪ¶Ø¹Ü£»¢Ę·¢ÉśµÄ·“Ó¦ŹĒ»ģŗĻĪļ£ØNa2OŗĶNa2O2£©Ź×ĻČÓėĖ®·“Ӧɜ³ÉĒāŃõ»ÆÄĘ£¬Č»ŗóŌŁŗĶŃĪĖį·¢ÉśÖŠŗĶ·“Ó¦£¬Ąė×Ó·½³ĢŹ½ĪŖNa2O+H2O=2Na++2OH- 2Na2O2+2H2O=4Na++4OH-+O2”ü H++OH-= H2O£»¢Ē¢ŚÖʱø¶žŃõ»ÆĢ¼£¬¢ÜĶعż¹żŃõ»ÆÄĘ¹ĢĢåÉś³ÉŃõĘų£¬¢Ż¶ŌŃõĘų½ųŠŠøÉŌļ£¬¢ŁÅÅĖ®·ØŹÕ¼ÆŃõĘų£¬¢ŪÓĆĮæĶ²ĮæČ”Ė®µÄĢå»ż¾ĶŹĒŃõĘųµÄĢå»ż”££Ø5£©·½°ø¶ž£ŗæÕĘųÖŠµÄŃõĘų»į¶Ō½į¹ū“ųĄ“øÉČÅ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com