
 ���һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��
���һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��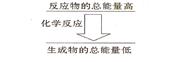


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
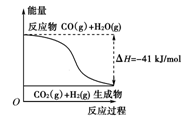
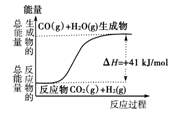
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)������H����41 kJ/mol |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol |
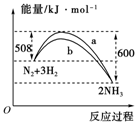
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH��l��+3/2O2��g��===CO2��g��+2H2O��l����H=��725.8 kJ/mol |
| B��2CH3OH��l��+3O2��g��===2CO2��g��+4H2O��l����H=��1452 kJ/mol |
| C��2CH3OH��l��+3O2��g��===2CO2��g��+4H2O��l����H=��725.8 kJ/mol |
| D��2CH3OH��l��+3O2��g��===2CO2��g��+4H2O��l����H=��1452 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���ձ�����������ĭ�����Ǽ���ʵ������е�������ʧ |
| B��ʹ�û��β������ȿ��Խ����ֱ������¶ȼ� |
| C����ʢװ����ձ��мӼ�ʱҪС�Ļ��� |
| D�����������¶ȼ�Ҫ��ˮ��ϴ���ٲ����¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼��Ʒֽ� | B�����������������Ȼ�茶��巴Ӧ |
| C��̼�������̼��Ӧ | D������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(l)DH=+28.7kJ/mol |
| B��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(1)DH=��28.7kJ/mol |
| C��NaOH(aq)+HCl(aq) = NaCl(aq)+H2O(1)DH=+57.4kJ/mol |
| D��NaOH(aq)+HCl(aq) = NaCl(aq)��H2O(1)DH=��57.4kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ɱ� | B��Һ�� | C������ | D��̼�ᱵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��2SO2+ O2 2SO3����H=��196.6kJ/mol 2SO3����H=��196.6kJ/mol |
| B��N2(g) + 2O2(g) ="=" 2NO2(g)����H= +67.7kJ/mol |
| C��C(s) + O2(g) ="=" CO2(g)����H=" +393.5kJ/mol" |
| D��H2O(l) ="=" H2(g)��+ 1/2O2(g)������H= +285.8kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2H2(g) + O2(g) ="=" 2H2O(1) ��H�� �D285.8kJ��mol |
| B��2H2(g) + O2(g) ="=" 2H2O(1) ��H�� +571.6 kJ��mol |
| C��2H2(g) + O2(g) ="=" 2H2O(g) ��H�� �D571.6 kJ��mol |
| D��H2(g) + O2(g) ="=" H2O(1) ��H�� �D285.8kJ��mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com