
������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K�� ��Na�� ��Fe2�� ��Ba2����NH |
| ������ | OH����NO |
(1)��A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ_________________________________________________________��
��A��B��Һ��Ϻ���ȳ����ԣ���Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��AΪ___________________________________________________________��
�ھ�����������������Һ��Ƶ�ԭ����������֣�
��.________________________________________________________________________��
��.________________________________________________________________________��
������һ������֤��������Һ��Ƶ�ԭ��__________________________________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪ
______________________________________________________________________ __
__
________________________________________________________________________��
������(1)����A��B�����ʺͷ�Ӧ�����֪��AΪNH4HSO4��BΪBa(OH)2������1��1��Ϸ�Ӧ�����Ⱥ���Һ�����ԣ���Ӧ�����ӷ���ʽΪ��H����SO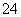 ��NH
��NH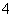 ��Ba2����2OH��
��Ba2����2OH��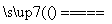 BaSO4����NH3����2H2O��
BaSO4����NH3����2H2O��
(2)��A��ˮ��Һ��dz��ɫ��˵��AΪ�����Σ�B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ��BΪ���Ρ���A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ�˵��BΪ�����ƣ���A��B��ˮ��Һ����������Ա仯֪��Aֻ����FeI2��
�𰸣�(1)��Ba(OH)2����H����SO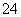 ��NH
��NH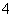 ��Ba2����2OH��
��Ba2����2OH��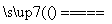
 BaSO4����NH
BaSO4����NH 3����2H2O[��Դ:ѧ&��&��]
3����2H2O[��Դ:ѧ&��&��]
(2)��FeI2���ڢ�.����I����������I2ʹ��Һ�ʻ�ɫ
��.I����Fe2����������ʹ��Һ�ʻ�ɫ
��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������(�������������)
��NO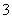 ��4H����3e��===NO����2H2O
��4H����3e��===NO����2H2O



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£���a L SO2��Cl2��ɵĻ������ͨ��100 mL 0.1 mol��L��1Fe2(SO4)3��Һ�У���ַ�Ӧ����Һ���ػ�ɫ��dz����Ӧ�����Һ�м���������BaCl2��Һ�������ó������ˡ�ϴ�ӡ��������أ�������Ϊ11.65 g�������й��ڸù��̵��ƶϲ���ȷ����(����)
A�����ó���Ϊ0.05 mol��BaSO4
B�����������SO2�����Ϊ0.448 L
C��a L�����������ʵ���Ϊ0.04 mol
D��a��ȡֵ��ΧΪ0.672<a<0.896
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж��������ʵ���������ȷ����(����)
A������ɫ B��������ˮ
C����Һ�� D����֧��ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��Ӿ��ˮ�еĺ�������Ӧ�ÿ�����0.5 mg��L��1��1.0 mg��L��1֮�䣬д����������ˮ�Ļ�ѧ����ʽ
________________________________________________________________________��
����ˮ�������________��ɱ��������
(2)��ͼ��ʾһ������ÿ��19ʱʱӾ��ˮ�е������������ļ���ʹ��Ӿ�ز���ȫ________________________________________________________________________��

(3)����Ϊ�ļ�����������ȡ�����ǿ��________��˵��һ��������______________��
(4)����һƬ��ɫ�Ļ��������ˮ�У��ɹ۲쵽��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ҵ����ظ����������������Һ���ܷ������·�Ӧ��C2H5OH��Cr2O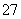 ��H���D��CO2����Cr3����H2O�����������ʽ��ƽ��H���Ļ�ѧ������Ϊ(����)
��H���D��CO2����Cr3����H2O�����������ʽ��ƽ��H���Ļ�ѧ������Ϊ(����)
A��10 B��12
C��14 D��16
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ����������������±���ʾ��
| ������ | NH |
| ������ | OH����NO |
ȡ�����������ֻ�����������ͬ�������Һ������ �����ʵ���Ũ�ȣ�c(��)>c(��)>c(��)���������ʿ�����(����)
�����ʵ���Ũ�ȣ�c(��)>c(��)>c(��)���������ʿ�����(����)
��MgSO4����NaOH����(NH4)2SO4����Mg(NO3)2����NH4NO3
A���٢� B���ۢ�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������(K2FeO4)��һ�����͡���Ч�������ɫˮ����������Cl2��O2��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
(1)�ɷ��Ʊ�������ص���Ҫ��ӦΪ��2FeSO4 �� 6Na2O2===2Na2FeO4 �� 2Na2O �� 2Na2SO4 �� O2��
�ٸ÷�Ӧ�е���������________����ԭ����________��ÿ����1 mol Na2FeO4ת��________mol���ӡ�
�ڼ�Ҫ˵��K2FeO4��Ϊˮ������ʱ���������__________________________________
________________________________________________________________________��
(2)ʪ���Ʊ��������(K2FeO4)�ķ�Ӧ��ϵ�����������ӣ�Fe(OH)3��ClO����OH����FeO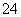 ��Cl����H2O��
��Cl����H2O��
��д������ƽʪ���Ƹ�����ط�Ӧ�����ӷ���ʽ��____________________________
________________________________________________________________________��
��ÿ����1 mol FeO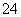 ת��________mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ________mol��
ת��________mol���ӣ�����Ӧ������ת����0.3 mol���ӣ���ԭ��������ʵ���Ϊ________mol��
�۵����£��ڸ���������Һ�м���KOH�����Ϳ������������(K2FeO4)��˵��ʲô����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʢ��NH4HCO3��ĩ��С�ձ�����ʢ����������Ĵ��ձ��С�Ȼ����С�ձ��м������ᣬ��Ӧ���ң����������̡��ɴ˿ɼ�(����)
A�� NH4HCO3������ķ�Ӧ�Ƿ��ȷ�Ӧ
B���÷�Ӧ�У�����ת��Ϊ�����ڲ�������
C����Ӧ��������������������������
D����Ӧ���Ȼ�ѧ����ʽΪ��NH4HCO3��HCl===NH4Cl��CO2����H2O����H����Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���Ӧ����ʵ��װ�ã�����װ��δ���������е�ʵ�飬��������ģ� ��
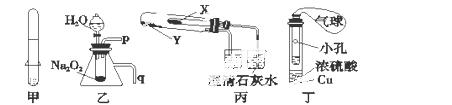
A�����ü�װ�ÿ�����ȡ����H2
B��������װ�ÿ�����֤Na2O2��ˮ��Ӧ�������������ַų�����
C�����ñ�װ����֤KHCO3��K2CO3�����ȶ��ԣ�X��Ӧ�ŵ�������K2CO3
D�����ö�װ����ȡSO2���������仹ԭ�ԣ�С�Թ��е��Լ���Ϊ����KMnO4��Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com