
��14�֣���ѧʵ�����о��������ʵĻ�����
��1�������й�ʵ�������������ݺ������� ������ţ���
a.������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b.�ø����pH��ֽ�ⶨŨ�����pH
c.�ù��Ϊ20mL����Ͳ����ȡ16.8mL��Na2CO3��Һ
��2��ij��ˮ��Ʒ�к���һ����Na+��CO32-��SO32-���ס������о�С�����ⶨ����SO32-���ӵ�Ũ�ȡ�
���鷽����
�Լ�X�������Լ���ѡ��
a.0.1mol��L-1KMnO4(H2SO4�ữ)��Һb.0.5mol��L-1NaOH��Һc.������ˮd.KI��Һ
�ټ�����Լ�XΪ ������ĸ��ţ�������SO42-��Ҫ�����ӷ���ʽΪ ��
�ڼ��鷽���У���iii���ġ�ϵ�в����������IJ������Ƹ�Ϊ ��
���鷽����
i.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ����ɫ�����ˣ�ȡ��Һ��
ii.��ȷ��ȡ20.00mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol��L-1KMnO4��H2SO4�ữ����Һ���еζ������йط�ӦΪ��2MnO4-+5SO32-+6H+=2Mn2++5SO42-+3H2O
iii.��¼���ݣ����㡣
��������Ƶ����еζ���ʽ�У���������� ���гֲ�����ȥ��������ĸ��ţ�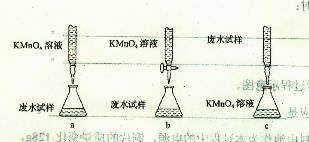
��3��ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ�ձ���ȡ��������ˮ���������ڣ����ձ��л����μӱ��͵�FeCl3��Һ���������ò����������������Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ���� ������Ϊ�ɹ��Ƶ�Fe(OH)3��������������� ��
��4������ͼװ�ý���CO2���ʵ��й�ʵ�顣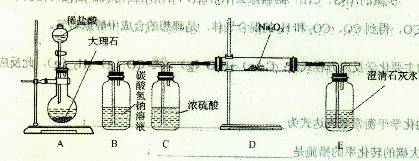
���Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ���� ��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧʵ�����о��������ʵĻ����������й�ʵ�������������ݺ������ǣ�
A��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
B���ø����pH��ֽ�ⶨŨ�����pH
C���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
D��KMnO4��ҺӦװ�ڼ�ʽ�ζ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧʵ�����о��������ʵĻ����������й�ʵ�������������ݺ������ǣ�
A��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
B���ø����pH��ֽ�ⶨŨ�����pH
C���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
D��KMnO4��ҺӦװ�ڼ�ʽ�ζ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�߶������ҵ��һ����ѧ�Ծ��������棩 ���ͣ�ʵ����
��ѧʵ�����о��������ʵĻ�����
(1)�����й�ʵ�������������ݺ�������________(�����)��
a��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b���ø����pH��ֽ�ⶨŨ�����pH
c���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
(2)ij��ˮ��Ʒ�к���һ������Na����CO32-��SO32-��ij�о�С�����ⶨ����SO32-��Ũ�ȡ�
ʵ�鷽����
��.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ�е����ʣ����ˣ�ȡ��Һ��
��.��ȷ��ȡ20.00 mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol/L KMnO4(H2SO4�ữ)��Һ���еζ���
��.��¼���ݣ����㡣
�����еζ���ʽ�У����������(�гֲ��ѷ���ȥ)______(����ĸ���)��

�ڵζ������У��йط�Ӧ�����ӷ���ʽ��__________________________________��
(3)ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ���ձ�ȡ��������ˮ���������ڣ����ձ��еμ� 1 mol/L��FeCl3��Һ���������ò��������裬�����Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ������������������������Ϊ�ɹ��Ƶ�Fe(OH)3���������������_________��
(4)����ͼװ�ý���CO2���ʵ��й�ʵ�顣

�Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ����:
_______________________ __________��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ��
____________________ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������2010��������Ĵ��ʼ쿼�ԣ���ѧ������ ���ͣ�ѡ����
��ѧʵ�����о��������ʵĻ����������й�ʵ�������������ݺ������ǣ�
A��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
B���ø����pH��ֽ�ⶨŨ�����pH
C���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
D��KMnO4��ҺӦװ�ڼ�ʽ�ζ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�{����һ������Կ��Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣���ѧʵ�����о��������ʵĻ�����
��1�������й�ʵ�������������ݺ������� ������ţ���
a.������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b.�ø����pH��ֽ�ⶨŨ�����pH
c.�ù��Ϊ20mL����Ͳ����ȡ16.8mL��Na2CO3��Һ
��2��ij��ˮ��Ʒ�к���һ����Na+��CO32-��SO32-���ס������о�С�����ⶨ����SO32-���ӵ�Ũ�ȡ�
���鷽����
�Լ�X�������Լ���ѡ��
a.0.1mol��L-1KMnO4(H2SO4�ữ)��Һb.0.5mol��L-1NaOH��Һc.������ˮd.KI��Һ
�ټ�����Լ�XΪ ������ĸ��ţ�������SO42-��Ҫ�����ӷ���ʽΪ ��
�ڼ��鷽���У���iii���ġ�ϵ�в����������IJ������Ƹ�Ϊ ��
���鷽����
i.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ����ɫ�����ˣ�ȡ��Һ��
ii.��ȷ��ȡ20.00mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol��L-1KMnO4��H2SO4�ữ����Һ���еζ������йط�ӦΪ��2MnO4-+5SO32-+6H+=2Mn2++5SO42-+3H2O
iii.��¼���ݣ����㡣
��������Ƶ����еζ���ʽ�У���������� ���гֲ�����ȥ��������ĸ��ţ�

��3��ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ�ձ���ȡ��������ˮ���������ڣ����ձ��л����μӱ��͵�FeCl3��Һ���������ò����������������Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ���� ������Ϊ�ɹ��Ƶ�Fe(OH)3��������������� ��
��4������ͼװ�ý���CO2���ʵ��й�ʵ�顣

���Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ���� ��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com