
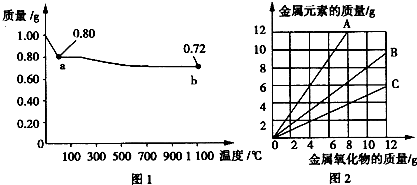
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98g���þ�����ƽ������Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����

A��ͼ1�У�A��B�Ĺ�������0.01 mol���ӷ�����ת��
B��ͼ1���������й�����0.26 gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A
D��ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO
A
��������
�����������0.98 gCu(OH)2��֪�����ʵ���Ϊ0.01 mol����ȫ������CuO��������Ϊ0.01 mol��80 g•mol-1��0.8g������A����CuO����ȫ������Cu2O��������Ϊ0.005 mol��144 g•mol-1��0.72g������B����Cu2O����D����ȷ��A��B�ķ�Ӧ������ͭ�ֽ�����������ͭ�����������ݻ�ѧ����ʽCu(OH)2 CuO+H2O��4CuO
CuO+H2O��4CuO 2Cu2O+O2����֪��0.26 g��ˮ�������������ͣ���B����CuO����������CuԪ�ص�������ϵ����CuO������Ϊ10g���㣩Ϊ�� CuO��Cu
2Cu2O+O2����֪��0.26 g��ˮ�������������ͣ���B����CuO����������CuԪ�ص�������ϵ����CuO������Ϊ10g���㣩Ϊ�� CuO��Cu
80 64
10g 8g
�۲�ͼ2��֪��B���߷�������������ϵ����ʾ����CuO����A���ϵ��κ�һ�㶼��ʾ���������������С��������������Ԫ�ص���������C��������ͭ�����ʵ�����0.01mol������ݷ�Ӧʽ4CuO 2Cu2O+O2����֪��A��B�Ĺ�������0.01 mol���ӷ�����ת�ƣ���A��ȷ����ѡA��
2Cu2O+O2����֪��A��B�Ĺ�������0.01 mol���ӷ�����ת�ƣ���A��ȷ����ѡA��
���㣺����������ͭ�ֽ���йؼ����Լ�ͼ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ����ʯ���С����ݸ��и���11��������ѧ�Ծ��������棩 ���ͣ�ѡ����
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98 g(�þ�����ƽ����)Cu(OH)2���壬���������ͭ�����������ɣ����������¶ȱ仯��������ͼ1��ʾ��
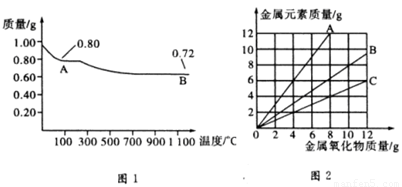
���⣬ijͬѧ������������ʾ����������������������Ԫ�������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26 g H2O
C��ͼ2���������У���ʾCuO����������CuԪ�������Ĺ�ϵ����������A
D��ͼ2�л��ƴ�������߹�2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ�����ڶ��νο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98g���þ�����ƽ������Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ���ǣ� ��
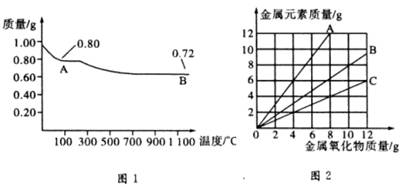
A��ͼ1�У�A��B�Ĺ�������0.01 mol���ӷ�����ת��
B��ͼ1���������й�����0.26 gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A
D��ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������һ��ģ���������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͭ�����ֳ����������CuO��Cu2O��ijѧϰС��ȡ0.98gCu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ��
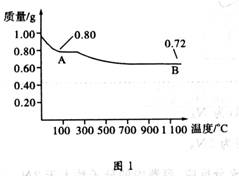
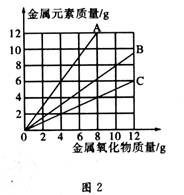
�����з�����ȷ���ǣ� ��
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������C
D��ͼ1�У�A��B��������0.01mol���ӷ�����ת��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com