
��ͼװ���У�A��B�е缫Ϊ��Ķ��Ե缫��C��DΪ����ʪ��Na2SO4��ֽ���ϵIJ��У�a��bΪ��Դ����.��A��B�г���KOH��Һ��ʹ�䵹����ʢ��KOH��Һ��ˮ����.�ж�K1���պ�K2��K3��ֱͨ���磬���һ��ʱ���A��B�в����������ȵ���ɫ���壬��ͼ��ʾ.
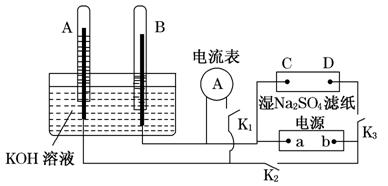
(1)�Թ�B���ռ��������� ���ѧʽ����Դ��a��Ϊ��������(�������������).
��2��д������C�Ϸ����ĵ缫��Ӧʽ��
��3��д��A�е缫��Ӧʽ�� .
(4)�����һ��ʱ���A��B�о��������Χ�缫.��ʱ�ж�K2��K3���պ�K1����������ָ�뷢��ƫת����ʱB���ĵ缫��ӦʽΪ ��.

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�| Fe |
| Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼװ���У�A��B�е缫Ϊ��Ķ��Ե缫��C��DΪ����ʪ��Na2SO4��ֽ���ϵIJ��У�a��bΪ��Դ����.��A��B�г���KOH��Һ��ʹ�䵹����ʢ��KOH��Һ��ˮ����.�ж�K1���պ�K2��K3��ֱͨ���磬���һ��ʱ���A��B�в����������ȵ���ɫ���壬��ͼ��ʾ.
(1)�Թ�B���ռ��������� ���ѧʽ����Դ��a��Ϊ��������(�������������).
��2��д������C�Ϸ����ĵ缫��Ӧʽ��
��3��д��A�е缫��Ӧʽ�� .
(4)�����һ��ʱ���A��B�о��������Χ�缫.��ʱ�ж�K2��K3���պ�K1����������ָ�뷢��ƫת����ʱB���ĵ缫��ӦʽΪ ��.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�������и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��18�֣���ͼװ���У�A��B������Һ�������Ϊ200mL��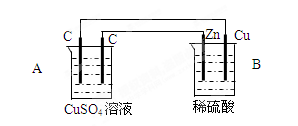
���ж�װ�õ����ƣ�A��Ϊ B��Ϊ
��A�������ʯī��Ϊ �����缫��ӦʽΪ
A���е���ܷ�Ӧʽ
(3)������һ��ʱ���B����Cu��������224ml���壨��״̬�������·��ͨ��
�� mol���ӣ�B������Һ���� ������ӡ��������䡱���١��� g������Ӧǰ����Һ��������䣬��Ӧ��A����Һ��pHΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��18�֣���ͼװ���У�A��B������Һ�������Ϊ200mL��
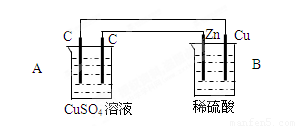
���ж�װ�õ����ƣ�A��Ϊ B��Ϊ
��A�������ʯī��Ϊ �����缫��ӦʽΪ
A���е���ܷ�Ӧʽ
(3)������һ��ʱ���B����Cu��������224ml���壨��״̬�������·��ͨ��
�� mol���ӣ�B������Һ���� ������ӡ��������䡱���١��� g������Ӧǰ����Һ��������䣬��Ӧ��A����Һ��pHΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com