
����10�֣�
��1����֪���淴Ӧ��M(g)��N(g) P(g)��Q(g)����H��0����ش��������⣺
P(g)��Q(g)����H��0����ش��������⣺
����Ҫ����M��ת���ʣ��������������������¿��Բ�ȡ�Ĵ�ʩΪ_______________��
A������һ����M B������һ����N C����Ӧ�¶�����
D����С������� E������ij���������� F�������һ����P
����ij�¶��£���Ӧ�����ʼŨ�ȷֱ�Ϊ��c(M)= 1 mol��L��1��c(N)=2.4 mol��L��1���ﵽƽ���M��ת����Ϊ60������ʱN��ת����Ϊ_______________���˷�Ӧ�Ļ�ѧƽ�ⳣ��K��_______________��
������Ӧ�¶Ȳ��䣬��Ӧ�����ʼŨ�ȷֱ�Ϊ��c(M)= 4 mol��L��1��c(N)=a mol��L��1���ﵽƽ���c(P)=2 mol��L��1��a=_______________mol��L��1��
��2�����ſ�ѧ�����Ľ������������Ƴ��˶������͵ļ״����ӽ���Ĥȼ�ϵ�أ������㲻ͬ������
��ͼ��ij�ʼDZ������ü״����ӽ���Ĥȼ�ϵ�صĽṹʾ��ͼ���״��ڴ����������ṩ���Ӻ͵��ӣ�����ת�Ƶķ�������ͼ��ʾ�������Ӿ����·�����Ӿ��ڵ�·������һ����������Ӧ������ܷ�ӦΪ��2CH3OH��3O2===2CO2��4H2O����c�缫�� �������������������c�缫�Ϸ����ĵ缫��ӦʽΪ_____________________________________________��

����10�֣�1����BCF(��1�֣���ѡ���ۣ�����Ϊֹ)
��25%��1�֣�K=0.5��1�֣���6��2�֣� ��2��������1�֣�CH3OH-6e-+H2O=CO2+6H+��2�֣�
����������1���ٷ�Ӧ���������ġ����ȵĿ��淴Ӧ������ѹǿ�ʹ������ܸı�ƽ��״̬��DE����ȷ������һ����M��ƽ��������Ӧ�����ƶ�����M��ת���ʽ��ͣ�A����ȷ������һ����N��ƽ��������Ӧ�����ƶ���M��ת��������B��ȷ�������¶�ƽ��������Ӧ�����ƶ���M��ת��������C��ȷ�������һ����P�������������Ũ�ȣ�ƽ��������Ӧ�����ƶ���M��ת��������F��ȷ�����Դ�ѡBCF��
��M��ת����Ϊ60����������M��0.6mol/L�����ݷ�Ӧʽ��֪������NҲ��0.6mol/L������N��ת������0.6��2.4��100%=25%������ͬʱ����P��Q��Ũ��Ҳ��0.6mol/L������ƽ�ⳣ������ ��
��
�۴ﵽƽ���c(P)=2 mol/L��������c(M)=2 mol/L, c(N)=2 mol/L������c(Q)=2
mol/L�����Ը���ƽ�ⳣ����֪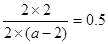 ���ǵ�a��6��
���ǵ�a��6��
��2��c�缫�ǵ��������ģ������Ǹ����������״�ʧȥ���ӣ��缫��ӦʽΪCH3OH-6e-+H2O=CO2+6H+��


 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��09-10��Ϸ����и�һ��ѧ����ĩģ�⿼�Ի�ѧ�� ���ͣ������
ұ�������������¼��ַ���������C��CO��H2����ԭ�� ���Ի��ý���Na��Mg�Ȼ�ԭ ���������ȷ�Ӧԭ����ԭ �ܵ�ⷨ ���ȷֽⷨ�����н������������ַ�����ԭ��ѡ����������д���пհס�������10�֣�
��1��Fe��Zn��Cu���еȻ��ý��� ��
��2��Na��Mg��Al�Ȼ��û�ϻ��ý��� ��
��3��Hg��Ag�Ȳ����ý��� ��
��4��V��Cr��Mn��W�ȸ��۵���� ��
��5��K��Rb��Cs��Ti�Ƚ���ͨ����ԭ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡܼ����У������ѧ�ڵڶ��ο��Ի�ѧ�Ծ����������� ���ͣ������
����10�֣�
��1����֪���淴Ӧ��M(g)��N(g) P(g)��Q(g)����H��0����ش��������⣺
P(g)��Q(g)����H��0����ش��������⣺
����Ҫ����M��ת���ʣ��������������������¿��Բ�ȡ�Ĵ�ʩΪ_______________��
| A������һ����M | B������һ����N |
| C����Ӧ�¶����� | D����С������� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮһ�и�һ5���¿���ѧ�Ծ����������� ���ͣ������
��д��C4H9Cl��ͬ���칹�壨ÿ��2�֣���10�֣�
��1�� ��2��
��3�� ��4��
��5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ���ƽ�ص�һ��ѧ�߶��ڶ��ε��п������ƻ�ѧ���⣨�������� ���ͣ������
��ÿ��2�֣���10�֣���1���ڴ���CuSO4��5H2O�����г���������Fe2+�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+���������ʿɲ��õ���_____
A��KMnO4 �� B�� H2O2 �� C�� Cl2ˮ ���� D�� HNO3
Ȼ���ټ��������ʵ����ʵ�������ҺpH=4��ʹFe3+ת��ΪFe(OH)3�����Դﵽ��ȥFe3+
������ʧCuSO4��Ŀ�ģ�������ҺpH��ѡ�������е�________
A. NaOH B. NH3��H2O C. CuO D. Cu(OH)2
��2����25 mL����������Һ����μ���0.2mol/L������Һ���õ���������ͼ��ʾ 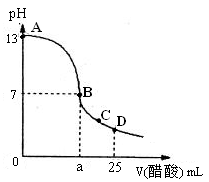
�١�д������������Һ�������Һ��Ӧ�����ӷ���ʽ
�ڡ�������������Һ�����ʵ���Ũ��Ϊ
�ۡ���B�㣬a 12.5 mL������ڡ�����С�ڡ����ڡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��³�ư���л�ѧѡ��5 1.1�л�������Ľṹ������ ����ϰ���������棩 ���ͣ�ʵ����
ʵ���⣨���10�֣�
��1������������Һ����������������������Һ ���Ҵ���ˮ ���廯�ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������� �� ��
A.��Һ����ȡ������ B.��ȡ������Һ
C.��Һ��������ȡ D.������ȡ����Һ
��2��ij��ѧ����С���ú���Ϊԭ����ȡ��������ˮ.����CCl4�ӵ�ˮ����ȡ�Ⲣ�÷�Һ©������������Һ.��ʵ������ɷֽ�Ϊ���¼���:
��A����50������ˮ��15����CCl4�����Һ©����,���Ǻò���������B����ʢ����Һ�ķ�Һ©����������̨����Ȧ�У���C������©��������,����ʱ������������,���رջ���,�ѷ�Һ©����������D�������Һ©���������ϿڵIJ������Ƿ�©Һ����E����������,���ջ�������Һ����G������,�ֲ㣨F������Һ©���Ͽڵ����ϲ�ˮ��Һ����H����©���ϿڵIJ�������ʹ���ϵİ��ۻ�С��©�����ϵ�С�ף��ʹ�ʵ�顣����������:
����ȷ���������˳����:�����������ٵı����ĸ��д��
���ߣߣߡ��ߣߣߡ��ߣߣߡ� �� ���ߣߣߣߡ� ��
��������E������IJ�����Ӧע�� ��������H�����������Ŀ���� ��
����ѡ��CCl4�ӵ�ˮ����ȡ���ԭ���� ��
����������,������Ϊ����ˮ����ȡ����ܼ����� ��
A���������� B���� C���ƾ� D��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com