
ʵ������Ҫ����0.50 mol·L��1 NaCl��Һ480 mL��
�����в������������ʵ������֣���ʹ��������������
(1)ѡ����������ɱ�ʵ��������������У�������ƽ��ҩ�ס��ձ���________��________��________�Լ�����������Ƭ��ֽ��
(2)���㡣���Ƹ���Һ��ȡNaCl����______ g��
(3)������
����ƽ��ƽ֮��Ӧ����ƽ���������ij��λ�ã�������ͼ����һ�����߱���������Ե������λ�ã�

�ڳ���������NaCl����Ӧ������ƽ��________(����̡������̡�)��
�۳�����ϣ���ҩƷ�����ձ��С�
(4)�ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����___________________________
________________________________________________________________________��
(5)ת�ơ�ϴ�ӡ���ת��ʱӦʹ�ò�������������Ҫϴ���ձ�2��3����Ϊ��________________________________________________________________________��
(6)���ݡ�������ƿ�м�ˮ��Һ��ӽ��̶���________��������________��ˮ��ʹ��Һ��Һ����̶������С�
(7)ҡ�ȡ�װƿ��
�𰸡�(1)500 mL����ƿ����ͷ�ιܡ���������(2)14.6
(3)��

������
(4)���裬����NaCl�ܽ⡡(5)��֤����ȫ��ת������ƿ��
(6)2��3 cm����ͷ�ι�
����������480 mL 0.50 mol·L��1��NaCl��Һ��������500 mL������ƿ��m(NaCl)��
0.50 mol·L��1��0.5 L��58.5 g·mol��1��14.6 g(������ƽ��ȷ��0.1 g)����������ƽ����ʱ����Ʒ�������̡�����һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ���������(����ȡ)���ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӵļ۵����Ų��У���Ӧ�ڵ�һ�����������ǣ� ����
A. 3s23p1 B. 3s23p2 C. 3s23p3 D. 3s23p4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�۲������Լ�ƿ�ϵı�ǩ���ش��������⡣

(1)��5%����ͭ��Һ���е�5%��ʲô���壿
(2)0.4 mol·L��1 NaCl��Һ�е�0.4 mol·L��1��ʾ�ĺ�����ʲô��
(3)������������Һ�зֱ�ȡ��5 mL������ͭ��Һ����������Ϊ__________��NaCl��Һ��Ũ��Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��100 gŨ��Ϊc mol·L��1���ܶ�Ϊ�� g·cm��3�������м���һ������ˮϡ�ͳ� mol·
mol·
L��1�����ᣬ�����ˮ�����________ 100 mL(���������������������ͬ)
(2)����(1)�е�H2SO4�ijɰ�ˮ��Ӧ����ˮ�����________ 100 mL��
(3)����(1)(2)�е����ʵ�����Ũ�Ⱦ���Ϊ���ʵ����������������ˮ�����________
100 mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����ε��ܽ��(S)��������ͼ��ʾ������˵������ȷ���� (����)

A����NaCl��Һ���ɿɵ�NaCl����
B����MgCl2��Һ���ɿɵ�MgCl2����
C��Mg(ClO3)2�л�������NaCl���ʣ������ؽᾧ���ᴿ
D������MgCl2��NaClO3�Ʊ�Mg(ClO3)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�ᾧˮ����Ļ�ѧʽΪR·nH2O������Է�������ΪM��25 ��ʱ����a g�þ�������
b gˮ��ǡ�ÿ��γ�V mL������Һ�����й�ϵ��ȷ���� (����)
A��������Һ�����ʵ���Ũ��
c�� mol·L��1
mol·L��1
B��������Һ�����ʵ���������w�� %
%
C��25 ��ʱR���ܽ��S�� g
g
D��������Һ���ܶ����� g·L��1
g·L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����з��ӽṹ�У�ԭ�ӵ��������Ӳ�������8�����ȶ��ṹ���ǣ� ��
A.CO2 B.PCl3 C.CCl4 D.NO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������л�Ϊͬλ�ص��� �� ��
A��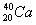 ��
��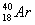 B��H2O��H2O2 C��D��T
B��H2O��H2O2 C��D��T D��O3��O2
D��O3��O2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com