
���� ��1��������Һ��������ж���Һ��pH��С����ͼ���Һ������ˮ�ĵ��룬����Һ�ٽ���ˮ�ĵ��룬�������Һ�������ӡ�����������Ũ��Խ��ˮ�ĵ���̶�ԽС���ݴ˽��н��
��2������̼��������Һ������Ũ�ȴ�С���бȽϣ������¶ȣ�NH4Cl��Һ��笠����ӵ�ˮ��̶�����
��3�����ݵ���ƽ�ⳣ������ʽ���õ�$\frac{c��N{{H}_{4}}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{Kb}{c��O{H}^{-}��}$��ͨ�백������Һ������������Ũ�����ñ�ֵ��С��
��4���ȱȽϵ������Ϻ���Һ������ԣ�Ȼ���ж���Һ��ʾ����ʱ���������ϵ��
��� �⣺��1����ͬŨ�ȵ���Һ�У���H2SO4��ҺΪ������Һ����NaHCO3��Һ��̼������Ӳ���ˮ�⣬��Һ��ʾ�����ԣ���NH4Cl��Һ������Ӳ���ˮ�⣬��Һ��ʾ�����ԣ���NaOH��ҺΪǿ����Һ��������4����ҺpH�ɴ�С��˳��Ϊ���ܢڢۢ٣��ڢ�Ϊ���������������������ӵ�����Һ���ٽ���ˮ�ĵ��룬���٢ֱܷ�Ϊ��ͼ���Һ��������ˮ�ĵ��룬���Тٵ����������Ũ�ȴ��ڢܵ�������������ӣ����Ԣ���ˮ�ĵ���̶���С��
�ʴ�Ϊ���ܢڢۢ٣��٣�
��2��̼��������Һ�У�̼���������ˮ�⣬��Һ��ʾ���ԣ�̼��������Һ������Ũ�ȴ�С��ϵΪ��c��Na+����c��HCO3-����c��OH-����c��H+����c��CO32-���������¶ȣ�NH4Cl��Һ��笠����ӵ�ˮ��̶���������������ǿ��pH��С���ʴ�Ϊ��c��Na+����c��HCO3-����c��OH-����c��H+����c��CO32-������С��
��3�����ݰ�ˮ�ĵ���ƽ�ⳣ������ʽ���õ�$\frac{c��N{{H}_{4}}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{Kb}{c��O{H}^{-}��}$���ñ�ֵ����Һ����������������Ũ�ȱ仯�йأ�������������������Һ������������Ũ���������Դ˱�ֵ��С��
�ʴ�Ϊ����С��
��4�����Ȼ��������������Һ��Ũ�ȵ������ϣ�����ǡ�÷�Ӧ�����Ȼ��ƺͰ�ˮ����Һ��ʾ���ԣ���Ҫʹ��Һ��ʾ���ԣ����Ȼ�淋����Ӧ�ô�Щ��������������Һ���СЩ�����Ȼ����Һ�����������������Һ�����
�ʴ�Ϊ�����ڣ�
���� ���⿼����ˮ�ĵ��롢������ʵĵ���ƽ�⡢�ε�ˮ��ԭ��������Ũ�ȴ�С�Ƚϵ�֪ʶ����Ŀ�Ѷ��еȣ�ע����ȷ��Һ���������ҺpH�Ĺ�ϵ�����㷽��������Ӱ��ˮ�ĵ��롢�ε�ˮ������أ��ܹ����õ���غ㡢�����غ�ȱȽ���Һ������Ũ�ȴ�С��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֱ������1��100 nm֮������ӳ�Ϊ���� | |
| B�� | ��Ӿ�����֤��������� | |
| C�� | 1 mol•L-1����������Һ��1 mol•L-1������������Һ���ֱ���������ʵ�飬������Һ�ĵ����Բ�ͬ | |
| D�� | �������������ܵ��磬���������������ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȣ��棩 | 10 | 20 | 30 | ������к���ȴ��50�� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
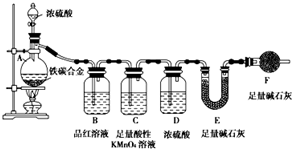 ��������̼����Ԫ����ɵĺϽ�ijʵ��С��Ϊ���о��úϽ�����ʲ��ⶨ�úϽ���̼���������������������ʵ�鷽����ʵ��װ��
��������̼����Ԫ����ɵĺϽ�ijʵ��С��Ϊ���о��úϽ�����ʲ��ⶨ�úϽ���̼���������������������ʵ�鷽����ʵ��װ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2���γ��������Ҫ���� | |
| B�� | CO2��������ЧӦ����һ�ִ�����Ⱦ�� | |
| C�� | CO2��g��+C��s��$\stackrel{����}{?}$2CO��g����H��0�����������ڸ÷�Ӧ�Է����� | |
| D�� | ʵ���ҳ��ô���ʯ��ϡ�����ϡ���ᷴӦ��ȡ������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���������ͼ�ش�
���������ͼ�ش�
 ��
�� ����
���� ��
�� +C2H5OH
+C2H5OH�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
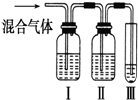 Ϊ̽����ϩ����ļӳɷ�Ӧ����ͬѧ��Ʋ�����������ʵ�飺�����Ҵ���Ũ����Ϊԭ����ȡ��ϩ�������ɵ�����ֱ��ͨ����ˮ�У�������Һ��ɫ����֤����ϩ����ˮ�����˼ӳɷ�Ӧ����ͬѧ�����ڼ�ͬѧ��ʵ���У������������д̼�����ζ���Ʋ����Ƶõ���ϩ�л����ܺ��������л�ԭ�Ե��������壬�ɴ�����������Ȱ����������ȥ��������ˮ��Ӧ��
Ϊ̽����ϩ����ļӳɷ�Ӧ����ͬѧ��Ʋ�����������ʵ�飺�����Ҵ���Ũ����Ϊԭ����ȡ��ϩ�������ɵ�����ֱ��ͨ����ˮ�У�������Һ��ɫ����֤����ϩ����ˮ�����˼ӳɷ�Ӧ����ͬѧ�����ڼ�ͬѧ��ʵ���У������������д̼�����ζ���Ʋ����Ƶõ���ϩ�л����ܺ��������л�ԭ�Ե��������壬�ɴ�����������Ȱ����������ȥ��������ˮ��Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com