
����̼������ͭ�ȳ���Ԫ���ڻ������г����ֳ����ּ�̬��������-2��-1��+2��+4��+6�ȼ�̬����ЩԪ���ڻ���������������ҪӦ�á�
��1��31.2gþ��̼�۵Ļ������һ��������ǡ����ȫ��Ӧ���ټ�������ˮ���õ�40.6g��ɫ������ͬʱ�����ܶ�Ϊ1.4107g/L����״�����ı���ϩ�Ͳ�������X�Ļ�����塣
��þ��̼�۵ķ�Ӧ����Ļ�ѧʽΪ ______________ ��
��ԭ�������̼�۵����ʵ�������Ϊ ___________������С����ʾ������2λС����
��2��ij������������Ԫ����ɣ������������Ӻ����������ӡ�ȡ21.76g�ø����Σ�ƽ����Ϊ���ݡ�����һ���������������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ�����ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ���������˺�����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣
�ٸø����εĻ�ѧʽΪ_____________��
�ڸø����������������ӵ�������Ϊ _________________ ��
��3��ij����ѧ�Ҿ����ⷢ��һ���µĴŻ�������Ҫ�ɷ�ΪFe1-xS1+x�����ʲ���Fe��S��������֪�ôŻ������У���Ԫ��������������������Ϊ75%��ȡ5�� 73%�ĸôŻ������ýӴ��������ᣬ������¯�����յ������Ϊ4%���Ӵ����з�Ӧ��ת����Ϊ94%��SO3������Ч��Ϊ97%���������Ƶ�98.3%��Ũ�����������________________��������2λС����
��1����MgC2��Mg2C3 ��0.63
��2����Cu5S(SO4)2 ��+1�۵�ͭ��+2�۵�ͭ=4:1
��3��6.08t
�������������
��1���ٸ��������֪��̼���V��Ӧ�õ��Ļ�������ˮ��Ӧ�IJ������ϩC3H4������ݻ��������������ϼ۵Ĵ�����Ϊ0��ȷ�������к���Mg2C3������ϩC3H4����Է�������Ϊ40������ϩ�Ͳ�������X�Ļ�������״�����ܶ�Ϊ1.4107g/L����ƽ����Է�������Ϊ1.4107��22.4=31.6.˵��������������Է�������С��31.6��ֻ��C2H2��C2H4�����ݻ��������������ϼ۵Ĵ�����Ϊ0��������C2H2��ȷ�������л����к���MgC2��������C2H4��ȷ�������л����к���MgC�����ʵ����ȥ�����þ��̼�۵ķ�Ӧ����Mg2C3��MgC2���ٹ������õ���Mg(OH)2�����ʵ���Ϊn(Mg(OH)2)="m��M=" 40.6g�� 58g/mol =0.7mol.����ԭ31.2gþ��̼�۵Ļ���ﺬ�е�C�����ʵ���Ϊn(C)=(31.2g��0.7mol�� 24g/mol) ��12 g/mol=1.2mol�� ԭ�������̼�۵����ʵ�������Ϊ1.2mol��(0.7+1.2)mol=0.63.
��2������һ�����������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ��֤������SO42-�������ʵ���Ϊn(SO42-)="9.32g��233g/mol=0.04mol;" ���ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ����������13.98g>9.32g֤��ԭ�����л�����S2-������������������������SO42-����n(SO42-) (��)="13.98g��233g/mol=0.06mol;" n(S2-)="0.06-0.04=0.02mol." ����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣֤������CuԪ��n(Cu2+)=" 8.00g��80g/mol=0.1mol." n(Cu2+): n(S2-): n(SO42-)=0.1:0.02:0.04=5:1:2.���Ը��εĻ�ѧʽΪCu5S(SO4)2 �ڸ�����Cu�Ļ��ϼ���+1��+2���ּ�̬������+1�۵�Ϊx����+2�۵�Ϊ(5-x).���ݻ��������������ϼ۵Ĵ�����Ϊ0�ɵ�x+2(5-x)=2+4.���x=4����5-x=1.����m(Cu+):m(Cu2+)=4:1.
��3���ôŻ������У���Ԫ��������������������Ϊ75%�������������������Ϊ25%��n(Fe3+):n(Fe2+)=3:1.���ݻ��������������ϼ۵Ĵ�����Ϊ0����ȷ����������S��ԭ�Ӹ���Ϊ3��3��1��2=11.���Ըû�����Ļ�ѧʽΪFe4S11.���������֪���ת��Ϊ�����S�����ʵ���Ϊ{(5��106g��73%��(1��4%)��94%��97%)��576g/mol}��11=6.1��104mol.���Եõ���98.3%��Ũ����������ǣ�6.1��104mol��98g/mol����98.3%=6.08��106g="6.08t" ��
���㣺�������ʻ�ѧʽ��ȷ������ɳɷֵĺ�����ԭ�ϵ������ʡ���Ʒ�IJ��ʵļ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ͬ״���µ�12C18O��14N2�������壬����˵����ȷ���� �� ��
| A����������ȣ������������ | B����ԭ������ȣ������������ |
| C������������ȣ��������� | D���������ȣ����ܶ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4molA�����2molB������2L���ܱ������л�ϣ���һ�������·������·�Ӧ��
2A(g)+B(g) 2C(g)������2�����C��Ũ��Ϊ0.6mol/L��
2C(g)������2�����C��Ũ��Ϊ0.6mol/L��
��1��2s����B��ʾ�ķ�Ӧ���� ��
��2��2sʱA�����ʵ���Ũ��Ϊ ��
��3��2sʱB�����ʵ���Ϊ ��
��4������C��Ũ�� ����ܡ����ܡ����ﵽ2mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��84����Һ������Чɱ�����H1N1������ijͬѧ������һƿijƷ�ơ�84����Һ����
������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25% NaClO��1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á������������Ϣ�����֪ʶ�ش��������⣺
(1)�á�84����Һ�������ʵ���Ũ��Ϊ________mol��L��1��
(2)��ͬѧȡ100 mL��Ʒ�ơ�84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na�� )��________mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)��ͬѧ���ĸ�Ʒ�ơ�84����Һ�����䷽������NaClO��������480 mL��25% NaClO������Һ������˵����ȷ����________��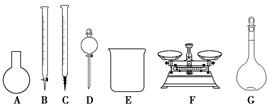
| A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ������� |
| B������ƿ������ˮϴ����Ӧ��ɲ���������Һ���� |
| C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ�� |
| D����Ҫ������NaClO��������Ϊ143 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��48 g RO42���У������������������������6.02��1023������Rԭ�ӵ�Ħ������Ϊ ��
����һ���ƿ������ΪM1 g����ƿ���������������ΪM2 g������ͬ״���£����ij�ij����A��������ΪM3 g����A����Է�������Ϊ ��
(2)һ������������������ȼ�գ����û������100 mL 3.00 mol��L��1��NaOH��Һ(�ܶ�Ϊ1.12 g��mL��1)ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.050 0 mol��
��ԭNaOH��Һ����������Ϊ ��
��������Һ��Cl�������ʵ���Ϊ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʼ�ת���Ŀ�ͼ���ش��й����⣻
`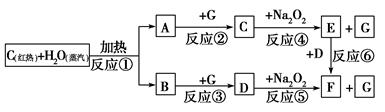
(1)�ɷ�Ӧ�ٲ�����A��B�����Ĺ�ҵ������________��
(2)д����ͼ��D��E�Ļ�ѧʽ��D________��E________��
(3)���2 mol Na2O2������ˮ������Ӧ���ɵñ�״��������������________L��ͬʱ��Ӧ��ת�Ƶ���������____________��(NA��ʾ�����ӵ�����)
(4)���A��B�������7.8 g������G��ַ�Ӧ��ͨ������Na2O2�㣬��ʹNa2O2����________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)2 mol O3��3 mol O2������֮�� ��������֮�� ��ͬ��ͬѹ�µ��ܶ�֮�� ������ԭ����֮�� �����֮�� ��
(2)O3��Cl2�������Ƶ����ʣ�������������ˮ����������֪����������ʱ������ԭΪ��ͼ�̬������ͬ״����10 L O3�� L Cl2�����������൱��
(3)���廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��10 mL A���ȷֽ�����15 mL O2��10 mL F2����A�Ļ�ѧʽΪ ���ƶϵ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| | ���ʵ����ʵ��� Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
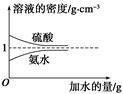
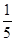 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________ 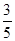 c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��ش��������⡣
��1��SO2���γ������������ס� 64 g SO2�����ʵ���Ϊ ���ڱ�״���µ����ԼΪ ������ԭ����Ϊ ��
��2��д����ȥ���������������ʣ�������Ϊ���ʣ����õ��Լ�����
��Na2CO3����(NaHCO3) ��FeCl3��Һ(FeCl2)
��Mg�ۣ�Al�� ��CO2(SO2)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com