
A��B��C������Ԫ�ص�ԭ�ӣ���������С��18��Aԭ����Bԭ�ӵ���������������6��Aԭ����Cԭ�ӵĺ�����Ӳ�����Ϊ3�� Cԭ�ӵ���������A��4���ش��������⣺
��1��A��B��Ԫ�ط��ŷֱ�Ϊ___________________��
��2��A�����ӽṹʾ��ͼΪ_________��A��C��ɵĻ�����Ϊ________���þ���Ԫ�ط��ű�ʾ����
��3��Ԫ��A��һ��������Ϊ32�ĺ���,��Ԫ��B��һ�ֺ��ع�����ΪAB42-��1mol AB42-������Ϊ104g,��Ԫ��B�ĸú����е�������Ϊ_________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017������ʡ�����и���ģ�⣨һ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�絼���Ǻ����������Һ����������С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣��һ���¶��£���0.1mol/LKOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol/L������ʹ�����Һ���ζ�������ͼ��ʾ�������й��ж���ȷ����
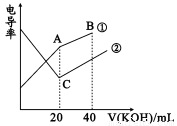
A. B�����Һ���У�c(K+)��c(OH-)>c(CH3COO-)>c(H+)
B. A�����Һ���У�c(CH3COO-)+c(OH-)-c(H+)=0.1mol/L
C. C��ˮ�����c(OH-)����A��ˮ�����c(OH-)
D. A��B��C������Һ����Kw=c(H+)��c(OH-)=1.0��10-14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�����и߶���ѧ��ѧҵˮƽ���ԣ�������ѧ�Ծ��������棩 ���ͣ�ѡ����
ij������Ʒ���ܺ���NH4HCO3��NH4C1��NH4NO3�е�һ�ֻ��֡���ȡ����Ʒ1.000�ˣ�����ˮ���100 mL��Һ������Һ�ֳ����ȷ������������ʵ�飺
����һ����Һ�м���10 mL 0.2 mol.L-1��������֮��ַ�Ӧ�����ռ�����״���µ�CO2����44.8 mL���������CO2ȫ���ݳ�����
������һ����Һ�м���������6 mol.L-1����������Һ�����ȣ����������壨�������NH3ȫ���ݳ���������Ҫ25 mL 0.15mol.L-1��������ܱ���ȫ��Ӧ������˵����ȷ����
A. 1.000 g��Ʒ��һ������NH4HCO3 0.316��
B. ��ٷ�Ӧ���õ���Һ�м��������ữ����������Һ�����а�ɫ�������ɣ�˵��ԭ��Ʒ��һ������NH4C1
C. ԭ��Ʒ�ĺ�NH4+����������Ϊ21%
D. �������������ʵ����֤������ȷ��ԭ��Ʒ���Ƿ���NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�����и߶���ѧ��ѧҵˮƽ���ԣ�������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼ��ʾʵ��װ�ã��г���������ȥ��̽��ͭ˿�����Ũ����ķ�Ӧ������ʵ�鲻��������
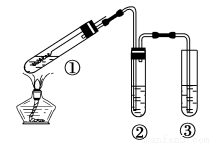
A. �����ƶ�����ͭ˿�ɿ���SO2�IJ�����ֹͣ
B. ����ѡ��Ʒ����Һ��֤SO2������
C. ����ѡ��NaOH��Һ���ն����SO2
D. Ϊȷ����CuSO4���ɣ�����м�ˮ���۲���ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����3�¸߿�ģ�⻯ѧ�Ծ��������棩 ���ͣ������
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ������й����ݻش��������⣺
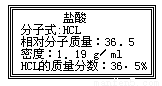
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________mol/L��
��2��ȡ����������ĸ�������Һʱ������������������ȡ ������ٶ��仯����____________��
A����Һ��H+�����ʵ���Ũ�� B����Һ��HCl������
C����Һ��H+����Ŀ D����Һ���ܶ�
��3��������1L1mol/L��ϡ���ᣬ��ʹ��Ũ������1������ȡ�Ĵ�ʩ���������_______��
A��ͨ������HCl����22.4L
B������Һ����Ũ����0.5L
C����ԭ��Һ����5mol/L����0.6L����ϡ����2L
D����ԭ��Һ����1L 3mol/L�����Ͼ��ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����3�¸߿�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�������������Խϴ�Ũ�ȹ�����ǣ� ��
A. ����0.1mol��L��1Fe3������Һ�У�K����Mg2����I����NO3��
B. ʹ��̪��Һ������Һ��Na����Cl����SO42����CO32-
C. ����0.1mol��L��1Ca2������Һ�У�Na����K����CO32����Cl��
D. ̼��������Һ��K����SO42����Cl����H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����3�¸߿�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����пƬ�ʹ�ͭƬ��ͼʾ��ʽ����ͬŨ�ȵ�ϡ������һ��ʱ�䣬����������ȷ���� �� ��
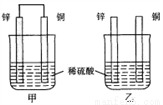
A. ���ձ�����Һ��pH������
B. ����ͭƬ������������ͭƬ�Ǹ���
C. ���ձ���ͭƬ����������ݲ���
D. �������ݵ��ٶȼױ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��������2����ѧ�Ծ��������棩 ���ͣ�ѡ����
W��X��Y��Z��ԭ��������������Ķ�����Ԫ�ء�m��n��p������ЩԪ����ɵĶ�Ԫ�����r��Ԫ��Y�ij������ʣ���ʹ�����ǵ�ľ����ȼ��q��ˮ��Һ�ʼ��ԣ�0��0l mol��L-l s��Һ��pHΪ12��q��s����ɫ��Ӧ���ʻ�ɫ���������ʵ�ת����ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
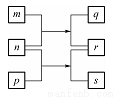
A. ԭ�Ӱ뾶��W<X<Y<Z
B. ͨ��״���£�X���⻯����ܳ���̬��Һ̬���̬
C. Y���⻯��ķе��Z���⻯��ķе��
D. ��W��X��Y��Z����Ԫ��ֻ�����һ�ֻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ̩���и�����һ�ָ�ϰ������⣨һģ�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
NA���������ӵ�������ֵ������������ȷ���ǣ� ��
A. 9 g����ˮ��3H216O����������Ϊ6NA
B. ��״���£�22.4 L CCl4���еķ�����ĿΪNA
C. ���³�ѹ�£�16g�����й��ۼ���ĿΪ4NA
D. 1 L 0.1 mol��L-1��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1 NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com