
��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
A��FeCl3 B��FeCl2 C��CuCl2 D��FeS
��2����ͬѧΪ��֤Fe�ܺ��ȵ�ŨHNO3��Ӧ�����������ͼ��ʾ��ʵ��װ�ã���˵��װ��B�����ã� ����ʼ����ǰ ����С����ޡ�����������
��3������ͬѧʵ�����ʱ������ȫ�ܽ⣬�Թ�Һ���Ϸ�Ϊ��ɫ���壬�Թ��ϲ�Ϊ����ɫ���壬��ʱ��ͬѧ�����õ���Һ��������ʵ���̽����
�������õ���Һ�м���һС��CuƬ�����CuƬ�����ܽ⣬��������������Ӧһ��ʱ������ܽ⡣��ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ������Ǻ�����HNO3������Ӧ�������ݵķ�Ӧ������ ���÷�Ӧ�����ӷ���ʽΪ ������ΪCuƬ�ܽ��Ƿ�����һԭ���������û�ѧ����ʽ��ʾ�����ɣ� �����˿ղ��
���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������ ��
�۱�ͬѧʵ��������ˮϡ�ͺ�õ���Һ500mL������ʵ��ȫ��������ֻ����ԭ��NO��
NO2��0.02 mol�������Һ��Fe2����Cu2+Ũ�Ⱦ�Ϊ0.02 mol/L����NO3-�����ʵ���Ũ��Ϊ
mol/L������NO��������Ϊ_________________L����״������
��4������̽��ʵ���õ��ܶ�Ϊ1.5g��cm-3��������Ϊ95%��Ũ����3mL������ʵ�ʲμӷ�Ӧ������ԭ���У�д�����㣩���� ��
��1��B��1�֣�
��2���ٻ������âڷ��������ã���1�֣��д������һ���1�֣��ޣ���1�֣�
��3����������ɫ���壻�Թ��ϲ����ֺ���ɫ���壨1�֣���
3Cu+2NO3-+8 H+=3Cu2++2NO��+4 H2O (2��)��2Fe(NO3)3+Cu=Cu(NO3)2+2Fe(NO3)2��2�֣���
��ȡ����Һ�������μ�������KSCN��Һ�������ֺ�ɫ���ٵμ�����������ˮ�����ֺ�ɫ��2�֣�
��0.08����2�֣�0.224����2�֣�
��4����Ũ�����ӷ���������ӷ���1�֣����������ȷֽ⡣��1�֣�
����:��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
A��FeCl3B��FeCl2 C��CuCl2D��FeS
��2����ͬѧΪ��֤Fe�ܺ��ȵ�ŨHNO3��Ӧ�����������ͼ��ʾ��ʵ��װ�ã���˵��װ��B�����ã� ����ʼ����ǰ ����С����ޡ�����������
��3������ͬѧʵ�����ʱ������ȫ�ܽ⣬�Թ�Һ���Ϸ�Ϊ��ɫ���壬�Թ��ϲ�Ϊ����ɫ���壬��ʱ��ͬѧ�����õ���Һ��������ʵ���̽����
�������õ���Һ�м���һС��CuƬ�����CuƬ�����ܽ⣬��������������Ӧһ��ʱ������ܽ⡣��ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ������Ǻ�����HNO3������Ӧ�������ݵķ�Ӧ������ ���÷�Ӧ�����ӷ���ʽΪ ������ΪCuƬ�ܽ��Ƿ�����һԭ���������û�ѧ����ʽ��ʾ�����ɣ� �����˿ղ��
���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������ ��
�۱�ͬѧʵ��������ˮϡ�ͺ�õ���Һ500mL������ʵ��ȫ��������ֻ����ԭ��NO��
NO2��0.02 mol�������Һ��Fe2����Cu2+Ũ�Ⱦ�Ϊ0.02 mol/L����NO3-�����ʵ���Ũ��Ϊ
mol/L������NO��������Ϊ _________________L����״������
��4������̽��ʵ���õ��ܶ�Ϊ1.5g��cm-3��������Ϊ95%��Ũ����3mL������ʵ�ʲμӷ�Ӧ������ԭ���У�д�����㣩���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�����һ��ѧ��һ��һѧ���������⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺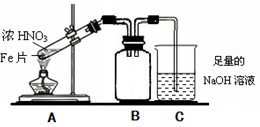
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
| A��FeCl3 | B��FeCl2 | C��CuCl2 | D��FeS |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����һ�и�һ��ѧ����ĩ������ѧ�Ծ���A������������ ���ͣ�ʵ����
��14�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧȡһ��ϸͭ˿��ɰֽ��ĥ���ھƾ����ϼ��������ȣ����쵽Cl2�ļ���ƿ�С�����Ϊ��ͬѧ��ʵ����Ӧ�ù۲쵽��������__________________________��
��2����ͬѧΪ��֤Fe�����ŨHNO3�з����ۻ����ܺ��ȵ�ŨHNO3��Ӧ���������ͼ��ʾ��ʵ�飬����ָ�����е���������____________________________��_________________________����������������������ɫ��ѧ�ĽǶȳ�������װ��2����Ľ����飬ʹװ��2���ܿ��Ʒ�Ӧ�Ľ��У����ܼ��ٵ���������ŷţ�__________________________________________.
��3����ͬѧ��Fe��ŨHNO3��Ӧ�����Һ�м���һ��CuƬ��CuƬ���ܽ⣬��Ӧһ��ʱ������ܽ⣨Cu��ʣ�ࣩ��
�ٱ�ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ���Ǻ�HNO3������Ӧ�������ݵķ�Ӧ������___________��
�ڶ�ͬѧ��Ϊ��ͬѧ�Ľ��۲���ȫ��ȷ������������_________________________��
��4���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ��������ͨ���и�һ��ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
| A��FeCl3 | B��FeCl2 | C��CuCl2 | D��FeS |
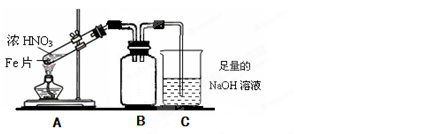
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ��һ��ѧ����ĩ������ѧ�Ծ���A���������棩 ���ͣ�ʵ����
��14�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧȡһ��ϸͭ˿��ɰֽ��ĥ���ھƾ����ϼ��������ȣ����쵽Cl2�ļ���ƿ�С�����Ϊ��ͬѧ��ʵ����Ӧ�ù۲쵽��������__________________________��
��2����ͬѧΪ��֤Fe�����ŨHNO3�з����ۻ����ܺ��ȵ�ŨHNO3��Ӧ���������ͼ��ʾ��ʵ�飬����ָ�����е���������____________________________��_________________________����������������������ɫ��ѧ�ĽǶȳ�������װ��2����Ľ����飬ʹװ��2���ܿ��Ʒ�Ӧ�Ľ��У����ܼ��ٵ���������ŷţ�__________________________________________.
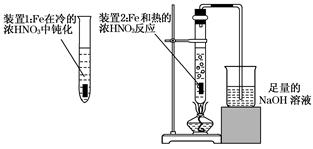
��3����ͬѧ��Fe��ŨHNO3��Ӧ�����Һ�м���һ��CuƬ��CuƬ���ܽ⣬��Ӧһ��ʱ������ܽ⣨Cu��ʣ�ࣩ��
�ٱ�ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ���Ǻ�HNO3������Ӧ�������ݵķ�Ӧ������___________��
�ڶ�ͬѧ��Ϊ��ͬѧ�Ľ��۲���ȫ��ȷ������������_________________________��
��4���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������_________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com