
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ�����Ʊ����������Ļ�ѧ����ʽ��CH3COOH��C2H5OH CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2 mL�Ҵ���2 mL���ᡢ1 mL 18 mol��L��1Ũ���� | ����̼������Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6mL 3 mol��L��1���� | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I����֪�л���A����̼���⡢��3��Ԫ�أ���������֪����Է�������Ϊ46���˴Ź� ��������ʾ�����������ֲ�ͬ��ѧ��������ԭ�ӣ��������µ�ת����ϵ��
��1����A��B�ķ�Ӧ������________
��2��D�ĺ�����ױ��������ڳ�CһH����CһC�����������CһO��������Ӧ���� D��HCl�����ʵ���֮��1:1��Ӧ����D�Ľṹ��ʽ�� ______
II ������E��F��ҩƷ��³����ϳɵ���Ҫԭ�ϣ���³����ĺϳ�·�����£���֪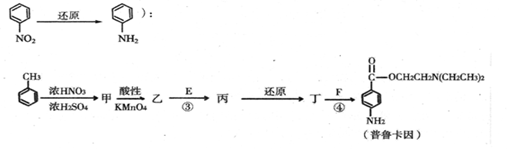
��3�����Ľṹ��ʽΪ_______________
��4����Ӧ�۵Ļ�ѧ����ʽ��__________________
��5����³����������ˮ�������ͼ��������������ͬ�ķ���ʽ�������������������ͬ���칹����______�֣������죩��
a�����ӽṹ�к���������ÿ��������2������
b�����ӽṹ��һ����������һNH2��һNH2ֱ����̼ԭ������
��6���쾭�ۺϷ�Ӧ�Ƴɵĸ߷�����ά�㷺����ͨѶ��������þۺϷ�Ӧ�Ļ�ѧ�� ��ʽ��__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij�л���Aֻ����һ����ԭ�ӡ���0.1 mol A��������������ȫȼ�ղ���3.6gˮ�����ﻹ����100 mL 2mol/L�ռ���Һǡ����ȫ��Ӧ����������Һ�ڵ��������ɣ��ܵõ�16.8g���������㣺��A�ķ���ʽ������A�ĺ˴Ź�������ֻ��һ�����շ壬��д��A�Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(10��)����������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
ʵ�鲽�裺
��A�м���4.4 g�����촼��6.0 g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143 ����֣�������������3.9 g��
�ش��������⣺
��1������B�������� ��
��2����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���� ���ڶ���ˮϴ����ҪĿ���� ��
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ�� �����ţ���
a��ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
��4����ʵ���м�����������Ŀ���� ��
��5��ʵ���м���������ˮMgSO4��Ŀ���� ��
��6������������У�����ѡ��װ����ȷ���� �����ţ��� ��7����ʵ��IJ����� �����ţ���
��7����ʵ��IJ����� �����ţ���
a��30�G b��40�G c��60�G d��90�G
��8���ڽ����������ʱ������130 ��㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ (��ߡ��͡�)�� ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(12��)ijʵ��С��������װ�ý����Ҵ���������ʵ�顣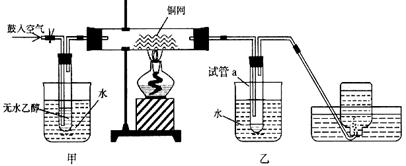
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ_________________��
��2����������ˮԡ���ò���ͬ���ҵ�������___________________������ƿ���ռ������������Ҫ�ɷ���_____________________��
��3�����Թ�a���ռ�����Һ������ɫʯ����ֽ����ֽ�Ժ�ɫ��˵��Һ���л���____________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���_________����д��ĸ����
| A���Ȼ�����Һ | B���� | C��̼��������Һ | D�����Ȼ�̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС���������������������װ�ã�����ͼ�����Ի������Ʊ�����ϩ


| | �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH CH3CH2CH2CHO
CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| | �е�/��c | �ܶ�/(g��cm-3) | ˮ���ܽ��� |
| ������ | 11.72 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
1��2-���������ڳ���������ɫҺ�壬�ܶ���2.18g/cm ���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ�����Ʊ�1��2-�������顣�����Թ�c��װ��Һ�壨���渲������ˮ����
���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ�����Ʊ�1��2-�������顣�����Թ�c��װ��Һ�壨���渲������ˮ����
��1��д���Ʊ�1��2-��������Ļ�ѧ����ʽ________��
��2����ȫƿa���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�c�е����Ƿ����������������������a�е�������__________��
��3������b��NaOH��Һ��������__________������d��NaOH��Һ��������__________��
��4���Թ�c������ˮ�У��Լ�Һ����渲������ˮ��ԭ����__________��
��5��ijͬѧ����ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ��ͨ�����ϩ��������������������³������ࡣ���װ�õ�������û�����⣬�Է������ܵ�ԭ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������ϩ�ļӳɲ������
| A��CH3CH3 | B��CH3CHCl2 | C��CH3CH2OH | D��CH3CH2Br |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com