
����Ŀ�������ķǽ���Ԫ�ؼ����������������������Ҫ��Ӧ�ã��밴Ҫ��ش�
��1��C��N��O��Si��S��Cl��Ԫ���γɵĵ�����
�����ڿ�����Ҫ�ɷ��������Ӧ�Ļ�ѧ����ʽ_____________________��
�ڳ���������ˮ��������ʵ�����Ʊ�������Ļ�ѧ����ʽ_____________________��
��2����CO��CO2��SO2��NO��NO2��SiO2����������
�����������������������______________������������_____________��
�����ά����Ҫ�ɷ����ռӦ�Ļ�ѧ����ʽΪ___________________��
��3����Ũ��ϡ�����ᡢŨ��ϡ�������У�
����������ʹFe��Al�ۻ�����_________��
�������ֽ�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ�_____________________��
��4����H2O2��NH3��HCl��
����������ɫ������������FeSO4������Һ��Ӧ�����ӷ���ʽΪ_______________________��
���������Ƶ��ʡ����������������������Ӧ�Ļ�ѧ����ʽΪ_________________��
���𰸡���1����N2+O2![]() 2NO��MnO2+4HCl��Ũ��
2NO��MnO2+4HCl��Ũ��![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��2����CO2��SO2��SiO2,SO2��NO��NO2��SiO2+2NaOH=Na2SiO3+H2O
��3����Ũ���ᡢŨ������4HNO3=4NO2��+O2��+2H2O
��4����2Fe2++H2O2+2H+=2Fe3++2H2O
��4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
��������
�����������1�������ڿ�����Ҫ�ɷ����������͵������������Ӧ�Ļ�ѧ����ʽΪN2+O2![]() 2NO��
2NO��
�ڳ���������ˮ��������������ʵ�����Ʊ�������Ļ�ѧ����ʽΪMnO2+4HCl��Ũ��![]() MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��
��2����CO��CO2��SO2��NO��NO2��SiO2���������У�������Ӧ�����κ�ˮ����������������������������������������CO2��SO2��SiO2�����γ��������SO2��NO��NO2�������ά����Ҫ�ɷ��Ƕ������裬���ռӦ�Ļ�ѧ����ʽΪSiO2+2NaOH=Na2SiO3+H2O��
��3����Ũ��ϡ�����ᡢŨ��ϡ�������У�
����������ʹFe��Al�ۻ�����Ũ���ᡢŨ���ᡣ
�������ֽ����Ũ���ᣬ��Ӧ�Ļ�ѧ����ʽ��4HNO3=4NO2��+O2��+2H2O��
��4����H2O2��NH3��HCl��
����������ɫ��������˫��ˮ������FeSO4������Һ��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+��2Fe3++2H2O��
���������Ƶ��ʡ�������ǰ�����������������������Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������״����飩�й����ʵ���Ũ�ȵļ���
��1����4gNaOH��������ˮ���250mL��Һ������Һ��NaOH�����ʵ���Ũ��Ϊ_________mol/L��ȡ��10mL����Һ�����к���NaOH_________g����ȡ������Һ��ˮϡ�͵�100mL��ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ_________mol/L��

��2����ͼʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��ݴ˼��㣺��Ũ������HCl�����ʵ���Ũ��Ϊ__________mol/L��������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol/L��ϡ���ᡣ��Ҫ��ȡ___________mL����Ũ����������ơ�
��3��100mL0.3mol/LNa2SO4��Һ��50mL0.2mol/LAl2(SO4)3��Һ��Ϻ���Һ��SO42�������ʵ���Ũ��Ϊ__________mol/L
��4����״���£���V L A���壨Ħ������ΪM g/mol������0.1Lˮ(�ܶ�1 g/cm3)�У�������Һ���ܶ�Ϊ![]() �������Һ�����ʵ���Ũ��Ϊ mol/L
�������Һ�����ʵ���Ũ��Ϊ mol/L
A��![]() B��
B��![]() C��
C��![]() D��
D��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� 1.52 gͭþ�Ͻ���ȫ�ܽ���50 mL�ܶ�Ϊ1.40 g/mL����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1 120 mL(��״��������Ӧ�����Һ�м���1.0 mol/L NaOH��Һ������������ȫ������ʱ���õ�2.54g����������˵������ȷ����
A���úϽ���ͭ��þ�����ʵ���֮����2:1
B����Ũ������HNO3�����ʵ���Ũ����14.0 mol/L
C��NO2��N2O4�Ļ�������У�NO2�����������80%
D���õ�2.54 g����ʱ������NaOH��Һ�������600 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������з�Ӧ���κ��¶��¾����Է����е���( )
A��2N2(g)��O2(g)===2N2O(g)��H����163 kJ��mol��1
B��Ag(s)��![]() Cl2(g)===AgCl(s)��H����127 kJ��mol��1
Cl2(g)===AgCl(s)��H����127 kJ��mol��1
C��HgO(s)===Hg(l)��![]() O2(g) ��H����91 kJ��mol��1
O2(g) ��H����91 kJ��mol��1
D��H2O2(l)=== ![]() O2(g)��H2O(l) ��H����98 kJ��mol��1
O2(g)��H2O(l) ��H����98 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ѧ�м��ֳ������ʵ�ת����ϵ��ͼ��ʾ��ͼ�в��ַ�Ӧ��������P��Ӧ����δ�г�����
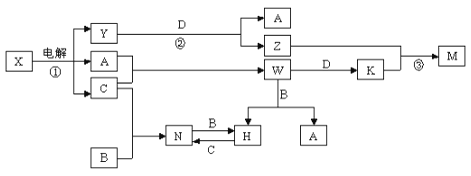
��֪��A��B��C��D�ǵ��ʣ������ǻ��������B��D�dz�����������֪A��C�е�ȼ�в�ɫ���棬M�ȿ����������ֿ�����NaOH��Һ����ش��������⣺
��1�� W�ĵ���ʽ��____________��Y�к��еĻ�ѧ������Ϊ____________��
��2��д��N�Ļ�ѧʽ__________������дһ��N����Ҫ��;_______________��
��3��д�����з�Ӧ�����ӷ���ʽ��
��Ӧ�� ��
��Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ѣ�CH3OCH3������Ϊ21���͵�����ȼ�ϣ�����ࡢ��Ч�����������Ļ������ܡ�
����д���пհף�
��l�����������Է������������Ԫ��������ͬ���л���Ľṹ��ʽ�ǣ�_____________��
��2�������ѿ��ɺϳ�����CO+H2����һ���������Ƶá��úϳ����ƶ�����ʱ����������һ�ֿɲ������ѭ������������÷�Ӧ�Ļ�ѧ����ʽ�����ǣ�______________________________��
��3���Զ����ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ�ǣ�_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��4- �����������������о��ۺ����ܼ���ϵ����ѧ���ʵ���Ҫ����,��ҵ�������з����Ʊ�1��4-���������飺
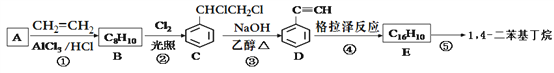
������ʾ��������Ӧ:2R��C��C��H ![]() R��C��C��C��C��R+H2
R��C��C��C��C��R+H2
�ش��������⣺
��1��D�Ĺ����ŵ�����Ϊ_____________________��
��2���ٺ͢۵ķ�Ӧ���ͷֱ�Ϊ____________��_____________��
��3��E�Ľṹ��ʽΪ_________________________________����1 mol E�ϳ�1��4�����������飬��������Ҫ��������________mol��
��4����д����Ӧ�ٵĻ�ѧ����ʽ______________________________��
��5��������C���������Ƶ�ˮ��Һ�м��ȿɵõ�������F,��д��������F�����ᷴӦ�Ļ�ѧ����ʽ______________________________________��
��6�����㻯����G��C��ͬ���칹�壬�������ֻ�����ֲ�ͬ��ѧ�������⣬��Ŀ��Ϊ1:1��д����ṹ��ʽ_______________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com