
ijС��ͬѧ���о�SO2�����ʡ�
��1������صĺ������ʷ�Ϊ���±���ʾ3�飬��2��������X�Ļ�ѧʽ�������������� �� ��
��1�� | ��2�� | ��3�� |
S�����ʣ� | SO2��X��Na2SO3��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |
��2��������ͼ��ʾ��װ���о�SO2�����ʣ�
���۵㣺SO2 ��76.1�棬SO3 16.8�棻�е㣺SO2 ��10�棬SO3 45�棩
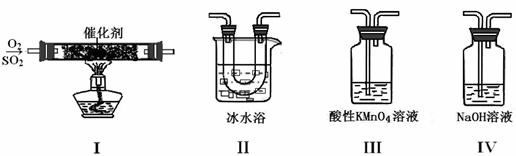 |
��װ��Iģ�ҵ������SO2�������ķ�Ӧ���仯ѧ����ʽ������������������������������������������������������������ ��
�ڼ�ͬѧ��I��II��III��IV��˳������װ�ã�װ��II������������������������������������������������������������������������������������ ��װ��III����Һ����ɫ������Mn2+��ͬʱpH���ͣ���÷�Ӧ�����ӷ���ʽ���������������������������������������� ���� ��
����ͬѧ��I��II��IV��˳������װ�ã���װ��IV����40 mL 2.5 mol?L��1 NaOH��Һ����Ӧ������4.8 g����װ��IV�з�����Ӧ�Ļ�ѧ����ʽ�������������������������������������������������������������������������������� �� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ��ɳ�и����������¿���ѧ�Ծ��������棩 ���ͣ������
��1��ij��ѧ��ȤС���ͬѧ����Cl2��NH3���Ʊ������ʼ����ʵ������̺Ͳ���װ�����£�
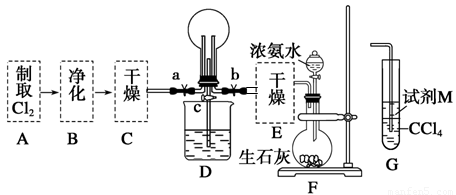
������A��Gװ�����һ����ʵ����֤Cl2��Fe3����I2��������ǿ��ΪCl2>Fe3��>I2(ʵ���в��ϵ�С����Gװ���е��Թ�)��A�з�Ӧ����KMnO4��Ũ���ᣬ��д��A�з�����Ӧ�Ļ�ѧ����ʽ�� ����д���Լ�MΪ ��Һ��֤��������ΪCl2>Fe3��>I2��ʵ�������� ��
����֪3Cl2��2NH3=6HCl��N2����D����ƿ�г�������ɫ����ر�a��c��b��D�е�����Ϊ����ɫ������ʧ���������̣���Ӧһ��ʱ��ر�b��c���۲쵽������Ϊ_________________________________________________________________��
��2��ij��ˮ�к���һ������Na+��SO �����ܺ���CO
�����ܺ���CO ��ij�о�С�����ⶨ����SO
��ij�о�С�����ⶨ����SO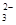 ��Ũ�ȣ��������ʵ�鷽����
��Ũ�ȣ��������ʵ�鷽����

�ٴ������Լ���ѡ���Լ�XΪ_________������ţ���
A��0.1 mol/L KMnO4(H2SO4�ữ)��Һ B��0.5 mol/L NaOH��Һ
C��������ˮ D��KI��Һ
�ڼ����Լ�X����SO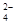 �����ӷ���ʽΪ_____________________________________��
�����ӷ���ʽΪ_____________________________________��
��֤���÷�ˮ���Ƿ���CO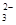 ��ʵ�鷽��Ϊ
��
��ʵ�鷽��Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ��ɳ�и����������¿���ѧ�Ծ��������棩 ���ͣ������
��1��ij��ѧ��ȤС���ͬѧ����Cl2��NH3���Ʊ������ʼ����ʵ������̺Ͳ���װ�����£�
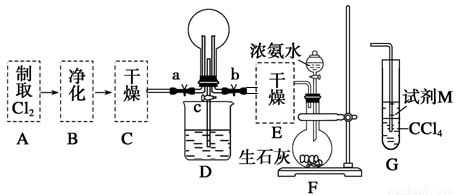
������A��Gװ�����һ����ʵ����֤Cl2��Fe3����I2��������ǿ��ΪCl2>Fe3��>I2(ʵ���в��ϵ�С����Gװ���е��Թ�)��A�з�Ӧ����KMnO4��Ũ���ᣬ��д��A�з�����Ӧ�Ļ�ѧ����ʽ�� ����д���Լ�MΪ ��Һ��֤��������ΪCl2>Fe3��>I2��ʵ�������� ��
����֪3Cl2��2NH3=6HCl��N2����D����ƿ�г�������ɫ����ر�a��c��b��D�е�����Ϊ����ɫ������ʧ���������̣���Ӧһ��ʱ��ر�b��c���۲쵽������Ϊ_________________________________________________________________��
��2��ij��ˮ�к���һ������Na+��SO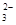 �����ܺ���CO
�����ܺ���CO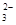 ��ij�о�С�����ⶨ����SO
��ij�о�С�����ⶨ����SO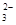 ��Ũ�ȣ��������ʵ�鷽����
��Ũ�ȣ��������ʵ�鷽����

�ٴ������Լ���ѡ���Լ�XΪ_________������ţ���
A��0.1 mol/L KMnO4(H2SO4�ữ)��Һ B��0.5 mol/L NaOH��Һ
C��������ˮ D��KI��Һ
�ڼ����Լ�X����SO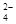 �����ӷ���ʽΪ_____________________________________��
�����ӷ���ʽΪ_____________________________________��
��֤���÷�ˮ���Ƿ���CO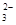 ��ʵ�鷽��Ϊ
��
��ʵ�鷽��Ϊ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com