
��12�֣�ijͬѧ���������ͼ��ʾװ�ã����ּг�װ������ȥ������ʵ���о���
��ش�
��1��������װ��̽��Ӱ�컯ѧ��Ӧ���ʵ����ء�
�� Բ����ƿ�з�����Ӧ�����ӷ���ʽ�� ��
�� ������װ�ý���ʵ�飬������9.0 mL����Ϊ��ʱ�յ㣬���Ϊt1��t2��
| ��� | V(H2SO4)/mL | c(H2SO4)/mol��L��1 | t/s |
| �� | 40 | 1 | t1 |
| �� | 40 | 4 | t2 |
�Ƚ�ʵ���͢���Եó���ʵ������� ��
ʵ������У��������ܵIJ����� ��
�� ����пƬ���ɺ����ʵĴ�пƬ���ҿ�����������ʹ��������ʵ����ȫһ�£�����õķ�Ӧ���ʾ���������ʵ���Ӧ�����ݡ���пƬ���������ʿ����ǣ�����ţ� ��
a��ʯī b���� c��ͭ d��ɳ�����������裩
��2��������װ����֤�����ڳ�ʪ�����лᷢ��������ʴ��
�� Բ����ƿ�е��Լ���ѡ�ã�����ţ� ��
a��NaOH��Һ b��C2H5OH c��NaCl��Һ d��ϡ����
�� ��֤�������ڳ�ʪ�����лᷢ��������ʴ�������� ��
 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

���������ش��������⣺
��1��ʵ�鲽���˳��ӦΪ������ţ�____________��
��2����a��b��c��ʾ�Ƶ����ԭ������Ϊ______________________________��
��3���������ض�ʵ�����ֱ��к�Ӱ�죿���ƫ��ƫС������Ӱ�족����û��Bװ��__________����ȡ�ý�����ʱδ����ú��__________���۳ƵõĽ����Ʊ���䰵��ŷ�����ƿ_______________���ܷ�Ӧʱ����ȼ������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��17�֣��ס��ҡ�������ѧ�г����ĵ��ʣ�X��Y��Z�dz����Ļ�����ڳ��³�ѹ�£����Ǿ��������ԵĻ���ɫ���壬�����غ�ɫ��Һ�壬Y��Z������ͬ�������ӣ�X��Z������ͬ�������ӣ�����֮��������ת����ϵ����ʮ��
��Z;Xʮ����Z��X+�ס�Y+������ش��������⣺
��1��д���ס��ҡ����������ʵĻ�ѧʽ___ _�� ��___ _��
��2��д��X�������ļ�����Һ����ȫ��Ӧʱ�����ӷ���ʽ ��
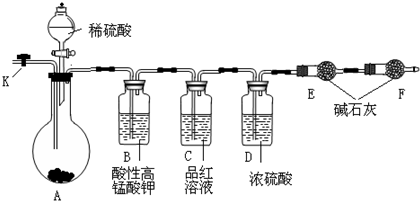
��3������ʵ��������ȡ���ռ����������������ף�Ȼ�����������Ӧ��X+�ס�Y+������ijͬѧ���������ͼ��ʾ��װ�á�
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��B�������� ��
��װ��C�е��Լ�Ϊ ��
��װ��D���ռ�����ķ��������� ��
��װ��F����Ҫ������ ����Ӧԭ�������ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡƽ��ɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
ijͬѧ���������ͼ��ʾ��װ�ý��е绯ѧʵ�飬�������װ��ͼ�ش�������⣺
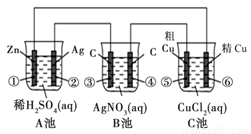
��1��C������ʲôװ�� ��
��2���缫���Ϸ����缫��ӦʽΪ ��B���з������ܷ�Ӧ����ʽΪ ��
��3����Ӧ����һ��ʱ���A��B��C�����е������ҺŨ�Ȼ����������________��
��4������·����0.2 mol��������ʱ���缫���������仯______g�� �缫���������仯______g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com