
 ijѧ��Ϊ��֤���ķе㣨80.1�棩��ˮ�ͣ��������ͼ1��ʾ��ʵ��װ�ã���ijЩ�̶�װ������ȥ��
ijѧ��Ϊ��֤���ķе㣨80.1�棩��ˮ�ͣ��������ͼ1��ʾ��ʵ��װ�ã���ijЩ�̶�װ������ȥ�� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������ĩ����������У�Fe��OH��3+3H+�TFe3++3H2O |
| B������������Һ��ͨ��������������Ca2++2ClO-+SO2+H2O�TCaSO3��+2HClO |
| C����̼��������Һ�еμӹ���������������2HCO3-+Ba2++2OH-�TBaCO3��+H2O+CO32- |
| D�����廯������Һ��ͨ������������2Fe2++Cl2�T2Fe3++2Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ʯ����̼ԭ�����γɵ���������ṹ��ռ�����������õ��ľ��пռ���״�ṹ��ԭ�Ӿ��壮���������У���һ̼ԭ��λ�����������ģ�������ֱ�����ڵ��ĸ�̼ԭ��λ�ڸ������廥�����ڵ��ĸ������ϣ���ͼ�е�С�����壩�����ʣ�ͼ����С�����嶥�ǵ��ĸ�̼ԭ��ֱ�����ڵ�̼ԭ����Ϊ���٣����Ƿֱ�λ�ڴ��������ʲôλ�ã�������
���ʯ����̼ԭ�����γɵ���������ṹ��ռ�����������õ��ľ��пռ���״�ṹ��ԭ�Ӿ��壮���������У���һ̼ԭ��λ�����������ģ�������ֱ�����ڵ��ĸ�̼ԭ��λ�ڸ������廥�����ڵ��ĸ������ϣ���ͼ�е�С�����壩�����ʣ�ͼ����С�����嶥�ǵ��ĸ�̼ԭ��ֱ�����ڵ�̼ԭ����Ϊ���٣����Ƿֱ�λ�ڴ��������ʲôλ�ã�������| A��12�����������12������е� |
| B��8�����������8������ |
| C��6�����������6��������� |
| D��14�����������8�����Ǻ�6��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HCN������ˮ |
| B��HCN��Һ�ܵ��� |
| C��1mol/L��������Һ��pHԼΪ3 |
| D��10 mL1 mol?L-1HCNǡ����10 mL 1 mol?L-1 NaOH��Һ��ȫ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ѧ��Ӧ���ʣ��ң��� |
| B��ƽ���N2��Ũ�ȣ��ң��� |
| C��H2��ת���ʣ��ң��� |
| D��ƽ��������H2������������ң��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� |
| �ŵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
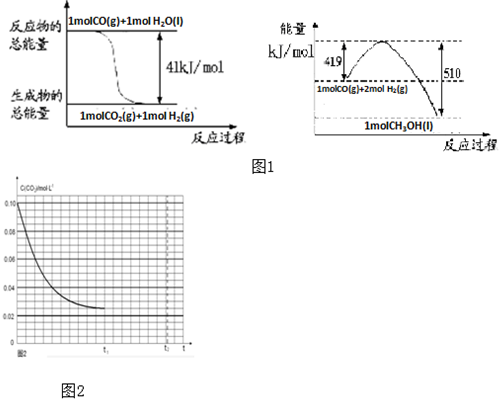
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
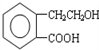 ����ͨ�����ò�ͬ��ѧ��Ӧ�ֱ��Ƶ�B��C��D �������ʣ�
����ͨ�����ò�ͬ��ѧ��Ӧ�ֱ��Ƶ�B��C��D �������ʣ�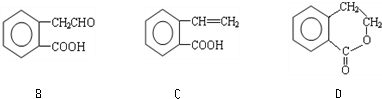
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com