
����Ŀ����֪ˮ��25 ���95 ��ʱ�ĵ���ƽ��������ͼ��ʾ��
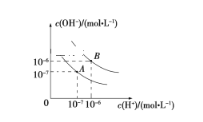
��1��25 ��ʱˮ�ĵ���ƽ������ӦΪ__________ (����A������B��)��
��2��95 ��ʱˮ�����ӻ�Kw=__________��cH��==__________
��3��25 ��ʱ����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7����NaOH��Һ��H2SO4��Һ�������Ϊ_____��
��4��95 ��ʱ����100 mL pH1=a��ijǿ����Һ��1 mL pH2=b��ijǿ����Һ��Ϻ�������Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��____��
��5��������B��Ӧ���¶��£���pH=2��ijHA��Һ��pH=10��NaOH��Һ�������ϣ�������Һ��pH=5���������ԭ��_________________��
���𰸡�A 1��1012 1��10-6mol/L 10��1 pH1+pH2=14 HAΪ����
��������
��1������ˮ�����ӻ�Kw=10��14����ˮʱ��ˮ�������c(H��)=c(OH��)=10��7mol��L��1����A���߱�ʾ25��ʱˮ�ĵ���ƽ�����ߣ�
��2������ͼ��95��ʱˮ�����ӻ�Kw=c(H��)��c(OH��)=10��6��10��6=10��12��H��Ũ��Ϊ10��6mol��L��1��
��3��25��ʱ�������Һ��pH=7����Һ�����ԣ���V(NaOH)��10��5=V(H2SO4)��10��4����V(NaOH)��V(H2SO4)=10��1��
��4��95��ʱˮ�����ӻ�Ϊ10��12����Һ�������ԣ���n(OH��)=n(H��)��100��10��3��10��a=1��10��3��10(b��12)��a��b=14����pH1��pH2=14��
��5������B����95��ˮ�ĵ���ƽ�⣬ˮ�����ӻ�Ϊ10��12����Һ�����ԣ�pH=6����pH=5ʱ����Һ�����ԣ�˵�����������HAΪ���ᡣ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��BΪԭ�Ϻϳɱ�����������F��·����ͼ��ʾ��

��1��A����ʽΪC2H2O3���ɷ���������Ӧ���Ҿ������ԣ�A��������������Ϊ__________��
��2��A+BC�ķ�Ӧ����Ϊ_______��A��ˮ��Һ����������������ͭ����Һ����,��Ӧ�Ļ�ѧ����ʽΪ_______��C( )������̼ԭ�������ӵ��ǻ��ڢ٢ڢ���������_______���ǿ������������
)������̼ԭ�������ӵ��ǻ��ڢ٢ڢ���������_______���ǿ������������
��3��B�����������_______��ԭ�ӹ��棬��B��ˮ��Һ�еμ�Ũ��ˮ������_______��F�ڼ���������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ_______��
��4��E����2����C���ɵĺ���3����Ԫ���Ļ����E���ӵĽṹ��ʽΪ_______��
��5���ڷ�������������F��ͬ���칹��(�����������칹)�У��˴Ź������������������ʵĽṹ��ʽΪ____________
������һԪ�������ڱ�����ֻ��2��ȡ�����Ҵ��ڶ�λ������һ�����ǻ���
��6����֪��![]() ��A�ж��ֺϳɷ������ڷ�����д��������ϳ�A��·������ͼ(����ԭ����ѡ)��_______
��A�ж��ֺϳɷ������ڷ�����д��������ϳ�A��·������ͼ(����ԭ����ѡ)��_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CH3COONa��Һ����������������ʹˮ��ƽ�������ƶ�������pH������ (����)
A. ����������CH3COOH
B. ��������NaCl����
C. ��������NaOH����
D. ��ˮϡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��г��ˢ�~������Ԫ�������ڱ��е�λ�ã�
�� ���� | ��A | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� |
(1)��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ_____________��
(2)��Ԫ��ԭ�ӵ�����������Ϊ___________��
(3)��Ԫ�����Ԫ���γɵ����ӻ�����Ļ�ѧʽΪ_________��д��һ�ּ��ɣ���
(4)�ڡ��ۡ�������Ԫ�صķǽ�������___________������������������ǿ������
(5)��Ԫ�غ͢�Ԫ���γɵ��⻯����ȶ��Ե�ǿ��˳��Ϊ_______��_______���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦA(g)��B(g) ![]() 2C(g)��3D(g)�����ֲ�ͬ����µķ�Ӧ�������£����б�ʾ
2C(g)��3D(g)�����ֲ�ͬ����µķ�Ӧ�������£����б�ʾ
��Ӧ����������
A. v(A)��0.20mol��L��1��min��1 B. v(B)��0.30 mol��L��1��min��1
C. v(C)��0.40 mol��L��1��min��1 D. v(D)��0.50 mol��L��1��min��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g)![]() CH3OCH3(g)��CO2(g) ��H��_________����ѧƽ�ⳣ��K��______________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
CH3OCH3(g)��CO2(g) ��H��_________����ѧƽ�ⳣ��K��______________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJmol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJmol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJmol-1 | K3 |
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g) ![]() CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%������H2��ʾ�ķ�Ӧ����Ϊ_________��CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%������H2��ʾ�ķ�Ӧ����Ϊ_________��CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч����D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ100mL��Na2CO3��Na2SO4�����Һ�����������BaCl2���29.02g��ɫ�������ù���ϡ���ᴦ������������ٵ�9.32g����������ų����Լ��㣺
(1)ԭ�����Һ��Na2CO3�����ʵ���Ũ��__________________��
(2)�����������ڱ�״���µ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ�����仯��AΪ������BΪ����ɫ���壬��ÿһ���ʾ�����AԪ�ء�

(1)���ƶ��������ʣ�
B.____________��C.____________��D.____________��E.____________���ѧʽ����
(2)д��B��C�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��p���ӵ�ԭ�ӹ����______�Σ�
��2�����ۼ������������ֱַ��������������������ĶԳƷ�ʽΪ______��
��3��ijԪ��λ��Ԫ�����ڱ��е������ڣ���VA�壬Ԫ�ط�����______������������Ӧ��ˮ����Ļ�ѧʽ______��
��4��������������������գ���������4p______5s�����Ӱ뾶��F-______Na+��
��5�����Ȼ�����S2Cl2���ǹ㷺������ҵ��������������һ�ֳȻ�ɫ�ж����Һ�壬���ķ��ӽṹ��ͼ��ʾ��
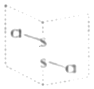
��S2Cl2�ĽṹʽΪ______���仯ѧ��������______���������Լ������Ǽ��Լ����������Լ��ͷǼ��Լ�������
�ڵ縺�ԣ�S______Cl����������������������ÿ��Sԭ����______�Ի����Ӷԡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com