
��8�֣�300����ǰ�������Ļ�ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����Ľṹʽ��ͼ��ʾ��
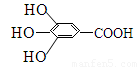
��1����ûʳ��������īˮ��Ҫ������_______________���������ʣ�����ţ���
A���� B���� C����֬ D������
��2��1 mol ûʳ������һ����������ȫȼ�գ��������������ʵ���Ϊ ��
��3��ûʳ����������п��������ã���Ŀǰ�㷺Ӧ�õ�ʳƷ���Ӽ�����ṹ��ʽΪ��
__ ______________________________ _
��4��д��ûʳ�������������������Һ��Ӧ�Ļ�ѧ����ʽ��
______________________ ___________
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 +CH3CH2OH+3CH3COONa
+CH3CH2OH+3CH3COONa +CH3CH2OH+3CH3COONa
+CH3CH2OH+3CH3COONa

| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ��ɽһ��2011��2012ѧ��߶���ѧ�����п��Ի�ѧ�������� ���ͣ�022
| |||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�300����ǰ�������Ļ�ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����Ľṹʽ��ͼ��ʾ��
��1����ûʳ��������īˮ��Ҫ������_______________���������ʣ�����ţ���
A���� B���� C����֬ D������
��2��1 mol ûʳ������һ����������ȫȼ�գ��������������ʵ���Ϊ ��
��3��ûʳ����������п��������ã���Ŀǰ�㷺Ӧ�õ�ʳƷ���Ӽ�����ṹ��ʽΪ��
__ ______________________________ _
��4��д��ûʳ�������������������Һ��Ӧ�Ļ�ѧ����ʽ��
______________________ ___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ��ɽһ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ������
��8�֣�300����ǰ�������Ļ�ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����Ľṹʽ��ͼ��ʾ��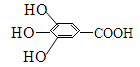
��1����ûʳ��������īˮ��Ҫ������_______________���������ʣ�����ţ���
| A���� | B���� | C����֬ | D������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com