
ijУ��ѧ�о���ѧϰС�������ʵ����֤Fe��Cu�Ľ�����ԣ��������
���������ַ��������������������й�ʵ����Ŀ��
�����������������С��ȵ���Ƭ��ͭƬ���ֱ�ͬʱ����ϡ����(��ϡ����)�У��۲�������ݵĿ������ݴ�ȷ�����ǵĻ�ԡ���ԭ�������ӷ���ʽΪ_______________________________________
��������������Fe��Cu���缫��Ƴ�ԭ��أ���ȷ�����ǵĻ�ԡ���������ķ����ڻ���ԭ���װ��ͼ�����ԭ��صĵ缫���Ϻ͵������Һ����д���缫��Ӧʽ��
������Ӧʽ��_______________________��
������Ӧʽ��_______________________��
�����������ѧ��֪ʶ���������������һ����֤Fe��Cu��Եļ�ʵ�鷽��(�뷽��������ͬ)��______________________�������ӷ���ʽ��ʾ�䷴Ӧԭ����________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(12��)ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����(FeSO4��7H2O)�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ������������������������������������
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�Ϻ�����;���������ǣ��� �������������� �� ���� ��������
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ��� ��
�� �⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
�⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ�
�������������������������������� ������������ �������� �������� ������������
��5��;���ܷ�����Ӧ�Ļ�ѧ����ʽΪ������������������ �� ������������������
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ������������������Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�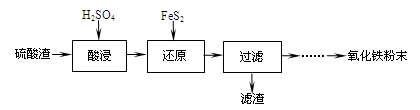
��1���������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ��� ��
��2������ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ
��
��3��Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��
ȷ��ȡһ���������������Һ����ƿ�У�����HCl���Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ����ʽ���£�
2Fe3����Sn2����6Cl����2Fe2����SnCl62����
Sn2����4Cl����2HgCl2��SnCl62����Hg2Cl2����
6Fe2����Cr2O72����14H����6Fe3����2Cr3����7H2O��
����SnCl2����������ⶨ��Fe3���� (�ƫ�ߡ�����ƫ�͡��������䡱����ͬ)��
��������HgCl2����ⶨ��Fe3���� ��
��4���ٿ�ѡ�� (���Լ�)������Һ�к���Fe3+������Fe3+��ԭ����
(�����ӷ�Ӧ����ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A���ĩ������B��ɵĻ�����ܷ�����ͼ��ʾ��һϵ�з�Ӧ��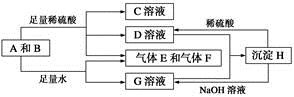
��ش��������⣺
(1)���A���ʵ�Ԫ�������ڱ��д��ڵ�__________����__________�塣
(2)������B�ĵ���ʽΪ_____________________________��
(3)D��G����Һ��Ϻ�����Ӧ�����ӷ���ʽΪ____________________
(4)�����£�D��Һ��pH________7(�����������������)����ԭ����____________________________(�����ӷ���ʽ��ʾ)��
(5)10.8 g A������������NaOH��Һ��Ӧ������������������Ϊ________ g��
(6)��̼����ϡ���ᡢ����E������F���ȼ�ϵ�أ��õ�ص�������ӦʽΪ______________________���Ըõ��Ϊ��Դ���ö��Ե缫���100 g 8%��C��Һ�������ʵ���������Ϊ12.5%ʱֹͣ��⣬��������У����ɵ�������״���µ������Ϊ________ L����·��ͨ�����ӵ����ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��I��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ��������Ҫ�����ɵ�ˮ���������ֲ�������ȥ����ش��������⣺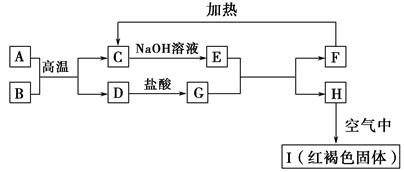
(1)B��F�ֱ��� (�ѧʽ)��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B�ڸ����·�Ӧ�� ��
��H�ڿ�����ת��ΪI�� ��
(3)E��Һ����������Ũ���ɴ�С��˳���� ��
(4)�����ӷ�Ӧ����ʽ��ʾG��Һ�����Ե�ԭ�� ���÷�Ӧ��ƽ�ⳣ��Ϊ (��֪�����£�H���ܶȻ�����Ksp��8.0��10��16)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ��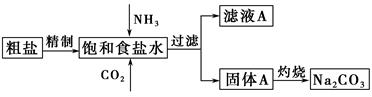
��֪����ʳ��ˮ��ͨ��NH3��CO2�����ķ�ӦΪNaCl��NH3��CO2��H2O=NaHCO3����NH4Cl����ش��������⣺
(1)�����к��е�����������Ca2����Mg2����SO42���ȡ�
���Ƴ��ӵIJ���˳����a�� �� �� ��b(����ĸ���)��
a�������ܽ⣬��ȥ����
b�����������pH
c������Ba(OH)2��Һ
d������Na2CO3��Һ
e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2�������� ��
(2)���չ���A��Na2CO3�� (����ĸ���)�н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4���ķ����� ��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH��13��Na����K������Һ�м�������NH4HCO3ʹpH���ͣ���Ӧ�����ӷ���ʽΪ ��
(3)��ͼװ���г�����ʵ�����Ʊ�CO2���� (����ĸ���)����bװ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ��� (���Լ�����)����ƿ�ڿɼ���Ĺ����Լ��� (���Լ�����)��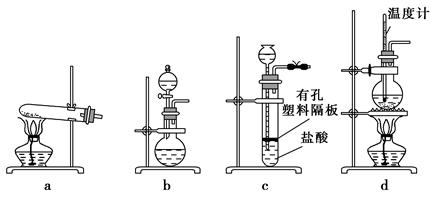
(4)һ����Ȼ���ɷ���aNa2CO3��bNa2SO4��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ�鷽����(������ѡ)���ʵ�鷽����ȫ��
��ѡ����Լ���1.0 mol��L��1 H2SO4��Һ��1.0 mol��L��1 BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1g��Ȼ�����Ʒ��������������ˮ�С�
�� ��
�� ��
�ܼ�����Ȼ����к�Na2CO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1862�꣬����ʱ��ѧ������ά�����˰���Ƽ1926�꣬�ҹ���ѧ�Һ�°�����
��Ϊ����°��Ƽ��Ҳ�������Ƽ�������Ƽ���������̿ɼ�Ҫ��ʾ����ͼ��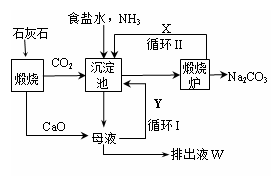

����������� �����Ƽ��������
��1�����������ͨ��CO2�Ͱ���ʱ��Ӧ��ͨ�백����ԭ���� ��
��2���������з�����Ӧ�Ļ�ѧ��Ӧ����ʽ�� �ӳ������з�������IJ����� ��
��3�������������ʾ��ͼ�е�Y�� ����ԭ�ϵ���Ʒ������ܷ�Ӧ�����û�ѧ����ʽ��ʾ����дΪ ��
��4�������Ƽ�д���Һ����ȡ�Ȼ�茶���Ĺ����Ʋ⣬���ý�����ȷ�� ��ѡ���ţ���
a������ʱ�Ȼ�淋��ܽ�ȱ��Ȼ���С
b��ͨ�백��������NH4+��Ũ�ȣ�ʹ�Ȼ�笠�������
c������ʳ��ϸ�������Na+��Ũ�ȣ� ʹNaHCO3�ᾧ����
d��ͨ�백����ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
��5�������Ƽ����ڰ�����Ȼ��������ʴ�70%��ߵ�90%���ϣ���Ҫ�������ѭ���������Ƽ����һ���ŵ��� ��
��6����Ʒ�����к���̼�����ƣ������ü��ȷֽ�ķ����ⶨ��Ʒ�д����������������֪��Ʒ����Ϊag���������������ٸı�ʱ����Ϊbg�������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
LiBH4Ϊ�������������������о��ȵ㡣
��1����Ӧ2LiBH4=2LiH��2B��3H2��������22.4 L H2(��״��)ʱ��ת�Ƶ��ӵ����ʵ���Ϊ mol��
��2����ͼ��2LiBH4/MgH2��ϵ�����ʱ�ʾ��ͼ����
Mg(s)��2B(s)=MgB2(s) ��H�� ��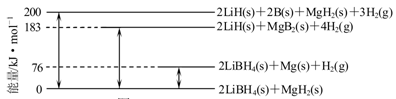
��3��������ĥ���Ʊ�Al��LiBH4�ĸ��ϲ��ϣ�����Al��LiBH4��ϵ��ˮ��Ӧ��������Խ��������о���
����ͼΪ25��ˮԡʱÿ�˲�ͬ��ȵ�Al��LiBH4���ϲ�����ˮ��Ӧ����H2�����ʱ��仯��ϵͼ����ͼ��֪������˵����ȷ���� (����ĸ)��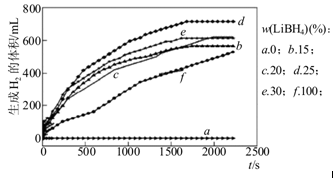
a��25��ʱ��������ˮ����Ӧ
b��25��ʱ����LiBH4��ˮ��Ӧ��������
c��25��ʱ��Al��LiBH4���ϲ�����LiBH4����Խ�ߣ�1000s�ڲ������������Խ��
����ͼΪ25���75��ʱ��Al��LiBH4���ϲ���[��(LiBH4)��25%]��ˮ��Ӧһ��ʱ�������X����������ͼ��(X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ)��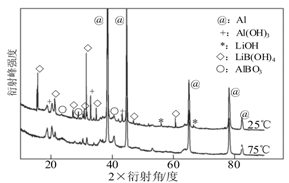
��ͼ�з�����25��ʱAl��LiBH4���ϲ�������ˮ��ȫ��Ӧ�������� (�ѧʽ)������Al(OH)3�Ļ�ѧ����ʽΪ ��
��4����ͼ��ֱ�����⻯�ƣ���������ȼ�ϵ��ʾ��ͼ���õ�ع���ʱ������������Һ��pH (���������С�����䡱)�������ĵ缫��ӦʽΪ ��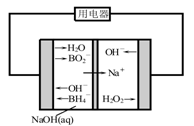
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��X��Ϊ��ѧ�����Ĵ��������֮������ͼת����ϵ(����������ȥ)��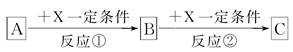
�ش��������⣺
(1)��X��ǿ�����Ե��ʣ���A��������________��
a��S b��N2 c��Na d��Mg e��Al
(2)��A��B��CΪ������Ԫ�ص��������XΪǿ�������Һ��A��Һ��C��Һ��Ӧ����B����B�Ļ�ѧʽΪ________��X�Ļ�ѧʽ����Ϊ(д����ͬ������)________��________����Ӧ�ٵ����ӷ���ʽΪ________________________��________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com