
��1������������ȼ�ϵ�صĹ���ԭ����ͼ��ʾ����֪����е����Ϊ���ڹ��������O2�����������������ƶ�����ȼ����ΪC2H4ʱ����������������Ӧʽ�ֱ�Ϊ
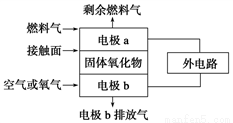
������______________________��
������____________________��
��2����ⷨ�������״���ˮ����ɵ���Ⱦ��ԭ���ǣ�ͨ�罫Co2��������Co3����Ȼ��Co3�����״�������CO2��H��(��ʯīϩ������ȥCo2��)��������ͼ��ʾװ��ģ���������̣���Co2���������ĵ缫��ӦʽΪ__________________����ȥ�״������ӷ���ʽΪ___________��
��3����ͼΪ��ͭ���ڳ�ʪ�����з����ĵ绯ѧ��ʴ��ʾ��ͼ��
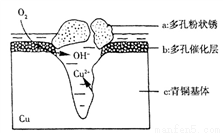
�ٻ����е�Cl����ɢ���ڣ�����������Ӧ���������Ӧ�����������ɶ��״��Cu2(OH)3Cl�������ӷ���ʽΪ______________________��
��������4.29 g Cu2(OH)3Cl���������Ϻ��������Ϊ___________L����״������
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����3�¸߿���Ӧ�Բ������ۻ�ѧ�Ծ��������棩 ���ͣ������
���ͪ��һ����Ҫ�Ļ���ԭ�ϣ�����ͨ�����¹��̺ϳɡ���ش������й����⣺
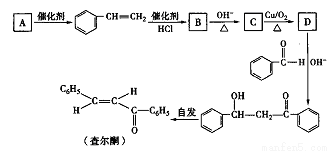
(1)��֪A�ķ���ʽΪC8H10,���Ľṹ��ʽΪ_______�����ͪ�Ľṹ����ϩ����_____ ���˳ʽ����ʽ�����칹��
(2) Bת����C�ķ�Ӧ��������________��C�����к���������Ϊ______��
(3)D������OH-����ʱ�뱽��ȩ��Ӧ�Ļ�ѧ����ʽΪ___________��
(4) D��ͬ���칹���У����ڷ���ȩ����_____�֣����к˴Ź����������շ��������ٵ�һ��ͬ���칹�������Ϊ________��
(5)���������ϳ�·�ߣ�д����CH3CH=CH2��ȡ��ͪ������ͼ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�������;��˵���������
A. SO2����Ѭ����˿ B. Al(OH)3������θ����кͼ�
C. AgI�����˹����� D. Fe2O3���������ɫ�����Ϳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
�л���W������������߷��Ӳ��Ϻϳɵ��м���ȣ��Ʊ�W��һ�ֺϳ�·�����¡�
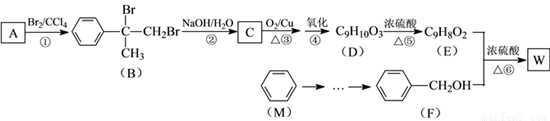
��֪��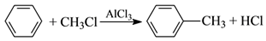
��ش��������⣺
��1��F�Ļ�ѧ������_________���ڵķ�Ӧ������_________��
��2��D�к��еĹ�������________________��д���ƣ���D�ۺ����ɸ߷��ӻ�����Ľṹ��ʽΪ_____________��
��3����Ӧ�۵Ļ�ѧ����ʽ��______________________��
��4����Ӧ�Ļ�ѧ����ʽ��______________________��
��5�����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ
_______________��
��6�������л���W�������ϳ�·�ߣ������MΪ��ʼԭ���Ʊ�F�ĺϳ�·��(���Լ���ѡ)��[ʾ���� ]
]
____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��M��WΪ���ֶ�����Ԫ�ء�X��Y��Z��ԭ���������ε�����ͬ����Ԫ�أ�������������֮��Ϊ15��X��Z���γ�XZ2���ӣ�Y��M�γɵ���̬�������ڱ�״���µ��ܶ�Ϊ0.76 g/L��W����������X��Y��Z��M����Ԫ��������֮�͵�1/2������˵���������
A. ԭ�Ӱ뾶��W��X��Y��Z��M
B. XZ2Ϊֱ���εĹ��ۻ�����
C. X��Y��Z �ֱ���MԪ���γɵ��������ķе���������
D. ��X��Y��Z��M����Ԫ���γɵĻ�����һ���������Ӽ����ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ڿ��淴ӦA2��g��+3B2��g�� 2AB3��g������H<0������ͼ����ȷ���ǣ�
2AB3��g������H<0������ͼ����ȷ���ǣ�
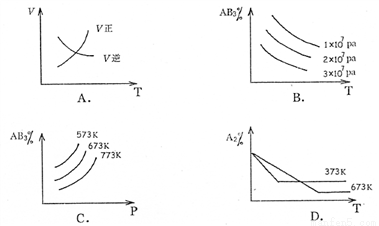
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��101kPa 25��ʱ��1.0g����������ȫȼ������Һ̬ˮʱ�ų�����52.0kJ��������ȼ�յ��Ȼ�ѧ����ʽΪ
A. C2H6(g) ��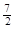 O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1
O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1
B. 2C2H6(g) �� 7O2(g)��4CO2(g) ��6H2O(g)��H =��1560kJ��mol��1
C. 2C2H6(g) �� 7O2(g)��4CO2(g) ��6H2O(l)��H =��3120kJ��mol��1
D. C2H6(g) ��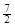 O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1
O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ʹCH3COONaϡ��Һ��c(CH3COO��)/c(Na��)��ֵ��������Һ��(����)�����������������еĢٹ���NaOH �ڹ���KOH���۹���CH3COONa���ܹ���NaHSO4
A. �ٻ�� B. �ڻ�� C. �ٻ�� D. �ۻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ����������������������ء�����˵����ȷ����
A. ����CO2���ŷţ����Լ�������IJ���
B. ����SO2���ŷţ����ԴӸ�������������
C. �õ�Ƴ��ķ�ˮֱ�ӹ��ũ������ˮ��������
D. ��CO2�ϳɾ�̼�����ɽ������ϣ�����ʵ�֡�̼����ѭ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com