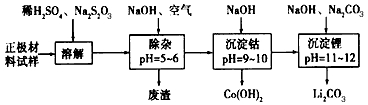
”¾“š°ø”æ
·ÖĪö£ŗ£Ø1£©¢Ł²»ÄÜÖ±½Ó»ģŗĻµÄŌŅņŹĒFe
2+ŌŚ¼īŠŌĢõ¼žĻĀøüČŻŅ×±»Ńõ»Æ£»
¢Śøł¾ŻĢāøųµÄŠÅĻ¢£¬·¢ÉśµÄ·“Ó¦ĪŖ£ØNH
4£©
2Fe£ØSO
4£©
2+LiOH+H
3PO
4=LiFePO
4+2NH
4HSO
4+H
2O£»
¢ŪĻūŗÄæÕĘųÖŠµÄO
2£¬±£»¤Fe
2+£¬·ĄÖ¹Fe
2+±»Ńõ»Æ£»
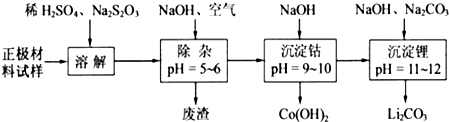
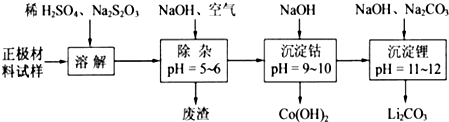
£Ø2£©¢ŁĶعżĢāøųŠÅĻ¢æÉÖŖLiCoO
2ÓėNa
2S
2O
3·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦£¬·“Ó¦ĪŖ8LiCoO
2+Na
2S
2O
3+11H
2SO
4=4LiSO
4+8CoSO
4+Na
2SO
4+11H
2O£»
¢Śøł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬ŌŚ±ä»Æ¹ż³ĢÖŠ£¬CoµÄÖŹĮæƻӊ±ä£¬ĶعżĢāøųŹż¾Żæ“£¬ŌŚ1000”ęŹĒCo£ØOH£©
2ĶźČ«·Ö½ā£¬Ōņ²śĪļCoO£»
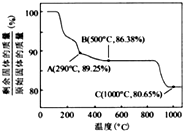
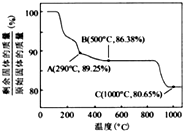
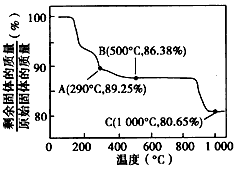
ŌŚ350-400”ꏱ£¬¹ĢĢåµÄÖŹĮæŌŚ89.25%-86.38%Ö®¼ä£¬æÉŅŌĶعż¼«µć½ųŠŠ·ÖĪö£¬
ŌŚ290”ę£¬n£ØCr£©£ŗn£ØO£©=
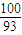
£ŗ[£Ø89.25-100×
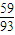
£©÷16]=2£ŗ3£¬Ęä»ÆѧŹ½ĪŖCo
2O
3£»
ŌŚ500”ęn£ØCr£©£ŗn£ØO£©=100/93£ŗ[£Ø86.38-100×
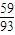
£©÷16]=3£ŗ4Ęä»ÆѧŹ½ĪŖCo
3O
4£¬
ĖłŅŌæÉŅŌČ·¶ØŌŚ350-400”ꏱµÄ»ÆѧŹ½ĪŖCo
2O
3ŗĶCo
3O
4£®
½ā“š£ŗ½ā£ŗ£Ø1£©£ØNH
4£©
2Fe£ØSO
4£©
2ŗĶLiOHČÜŅŗ·“Ӧɜ³ÉFe£ØOH£©
2£¬Fe£ØOH£©
2Ņ×±»ŃõĘųŃõ»Æ£»ĖłŅŌ²»Äܽ«Ö±½Ó»ģŗĻ£»¹Ź“š°øĪŖ£ŗFe
2+ŌŚ¼īŠŌĢõ¼žĻĀøüŅ×±»Ńõ»Æ£Ø·²ŗĻĄķ“š°ø¾łæÉ£©£»
¢Śøł¾ŻĢāøųŠÅĻ¢£ŗ£ØNH
4£©
2ӢH
3PO
4ÓėLiOHČÜŅŗ·¢Éś¹²³Įµķ·“Ӧɜ³ÉLiFePO
4ӢNH
4HSO
4ŗĶH
2O£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ£ØNH
4£©
2Fe£ØSO
4£©
2+LiOH+H
3PO
4=LiFePO
4+2NH
4HSO
4+H
2O£»¹Ź“š°øĪŖ£ŗ£ØNH
4£©
2Fe£ØSO
4£©
2+LiOH+H
3PO
4=LiFePO
4”ż+2NH
4HSO
4+H
2O£»
¢ŪøßĪĀ³ÉŠĶĒ°£¬³£ĻņLiFePO
4ÖŠ¼ÓČėÉŁĮæ»īŠŌĢæŗŚ£¬Ęä×÷ÓĆ³żĮĖæÉŅŌøÄÉĘ³ÉŠĶŗóµÄLiFePO
4µÄµ¼µēŠŌÄÜĶā£¬»¹ÄÜĻūŗÄæÕĘųÖŠµÄŃõĘų£¬±£»¤Fe
2+£¬·ĄÖ¹Fe
2+±»Ńõ»Æ£»¹Ź“š°øĪŖ£ŗÓėæÕĘųÖŠO
2·“Ó¦£¬·ĄÖ¹LiFePO
4ÖŠµÄFe
2+±»Ńõ»Æ£»
£Ø2£©Õż¼«²ÄĮĻŹŌŃłÖ÷ŅŖŗ¬ÓŠLiCoO
2¼°ÉŁĮæAl”¢FeµČ£¬¼ÓČėĻ”H
2SO
4ӢNa
2S
2O
3£¬S
2O
32-±»Ńõ»Æ³ÉSO
42-£¬¾ßÓŠ»¹ŌŠŌ£¬Õż¼«²ÄĮĻÖŠÖ»ÓŠLiCoO
2¾ßÓŠŃõ»ÆŠŌ£¬Óė·“Ó¦Na
2S
2O
3·“Ӧɜ³ÉCoSO
4£¬·“Ó¦»Æѧ·½³ĢŹ½ĪŖ£ŗ8LiCoO
2+Na
2S
2O
3+11H
2SO
4=4Li
2SO
4+8CoSO
4+Na
2SO
4+11H
2O£»¹Ź“š°øĪŖ£ŗ8LiCoO
2+Na
2S
2O
3+11H
2SO
4=4Li
2SO
4+8CoSO
4+Na
2SO
4+11H
2O£»
¢Śøł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬ŌŚ±ä»Æ¹ż³ĢÖŠ£¬CoµÄÖŹĮæƻӊ±ä£¬¼ŁÉčŌŹ¼¹ĢĢåÖŹĮæĪŖ100g£¬Ōņn£ØCo£©=
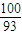
mol£¬m£ØCo£©=100×
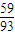
g£»
ŌŚ1000”ꏱ£¬¹ĢĢåÖŹĮæ²»ŌŁ±ä»Æ£¬ĖµĆ÷Co£ØOH£©
2ĶźČ«·Ö½ā£¬n£ØCr£©£ŗn£ØO£©=
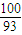
£ŗ[£Ø80.65-100×
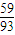
£©÷16]=1£ŗ1£¬Ź£Óą¹ĢĢå³É·ÖĪŖCoO£»
ŌŚ350-400”ꏱ£¬¹ĢĢåµÄÖŹĮæŌŚ89.25%-86.38%Ö®¼ä£¬æÉŅŌĶعż¼«µć½ųŠŠ·ÖĪö£¬
ŌŚ290”ę£¬n£ØCo£©£ŗn£ØO£©=
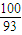
£ŗ[£Ø89.25-100×
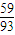
£©÷16]=2£ŗ3£¬Ęä»ÆѧŹ½ĪŖCo
2O
3£»
ŌŚ500”ęn£ØCo£©£ŗn£ØO£©=
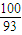
£ŗ[£Ø86.38-100×
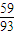
£©÷16]=3£ŗ4£¬Ęä»ÆѧŹ½ĪŖCo
3O
4£»
ĖłŅŌæÉŅŌČ·¶ØŌŚ350-400”ꏱµÄ»ÆѧŹ½ĪŖCo
2O
3ŗĶCo
3O
4£¬¹Ź“š°øĪŖ£ŗCoO£»Co
2O
3ӢCo
3O
4£®
µćĘĄ£ŗ±¾Ģāæ¼²ģµÄÖŖŹ¶±Č½ĻÉ¢£¬Éę¼°µ½ÄÜŌ“ĄūÓĆ£¬ĪļÖŹŠŌÖŹ”¢»Æ¹¤Į÷³Ģ·ÖĪö£¬Ķ¼±ķ·ÖĪö£¬ø²øĒĆę±Č½Ļ¹ć£®



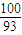 £ŗ[£Ø89.25-100×
£ŗ[£Ø89.25-100×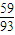 £©÷16]=2£ŗ3£¬Ęä»ÆѧŹ½ĪŖCo2O3£»
£©÷16]=2£ŗ3£¬Ęä»ÆѧŹ½ĪŖCo2O3£»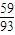 £©÷16]=3£ŗ4Ęä»ÆѧŹ½ĪŖCo3O4£¬
£©÷16]=3£ŗ4Ęä»ÆѧŹ½ĪŖCo3O4£¬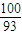 mol£¬m£ØCo£©=100×
mol£¬m£ØCo£©=100×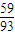 g£»
g£»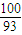 £ŗ[£Ø80.65-100×
£ŗ[£Ø80.65-100×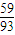 £©÷16]=1£ŗ1£¬Ź£Óą¹ĢĢå³É·ÖĪŖCoO£»
£©÷16]=1£ŗ1£¬Ź£Óą¹ĢĢå³É·ÖĪŖCoO£»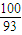 £ŗ[£Ø89.25-100×
£ŗ[£Ø89.25-100×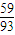 £©÷16]=2£ŗ3£¬Ęä»ÆѧŹ½ĪŖCo2O3£»
£©÷16]=2£ŗ3£¬Ęä»ÆѧŹ½ĪŖCo2O3£»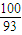 £ŗ[£Ø86.38-100×
£ŗ[£Ø86.38-100×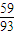 £©÷16]=3£ŗ4£¬Ęä»ÆѧŹ½ĪŖCo3O4£»
£©÷16]=3£ŗ4£¬Ęä»ÆѧŹ½ĪŖCo3O4£»