
ЁОЬтФПЁПЃЈIЃЉбаОПДѓЦјжаКЌСђЛЏКЯЮяЃЈжївЊЪЧ SO2 КЭ H2SЃЉЕФзЊЛЏОпгаживЊвтвхЁЃ
ЃЈ1ЃЉЙЄвЕЩЯВЩгУИпЮТШШЗжНтH2SЕФЗНЗЈжЦШЁH2ЃЌдкФЄЗДгІЦїжаЗжРыH2ЃЌЗЂЩњЕФЗДгІЮЊЃК 2H2S(g) ![]() 2H2(g)ЃЋS2(g) ІЄH
2H2(g)ЃЋS2(g) ІЄH
вбжЊЃКЂйH2S(g) ![]() H2(g)ЃЋS(g) ІЄH1ЃЛ Ђк2S(g)
H2(g)ЃЋS(g) ІЄH1ЃЛ Ђк2S(g) ![]() S2(g) ІЄH2ЁЃ
S2(g) ІЄH2ЁЃ
дђ ІЄHЃН________________(гУКЌ ІЄH1ЁЂІЄH2ЕФЪНзгБэЪО)ЁЃ
ЃЈ2ЃЉЭСШРжаЕФЮЂЩњЮяПЩНЋДѓЦјжа H2S ОСНВНЗДгІбѕЛЏГЩ SO42-ЃЌСНВНЗДгІЕФФмСПБфЛЏЪОвтЭМШчЯТЃК
1mol H2S(g)ШЋВПбѕЛЏГЩSO42-(aq)ЕФШШЛЏбЇЗНГЬЪНЮЊ________________ЁЃ
ЃЈIIЃЉ100ЁцЪБЃЌдк1LКуЮТКуШнЕФУмБеШнЦїжаЃЌЭЈШы0.1mol N2O4ЃЌЗЂЩњЗДгІЃКN2O4(g) ![]() 2NO2(g) ІЄHЃНЃЋ57.0kJЁЄmolЃ1ЃЌNO2КЭN2O4ЕФХЈЖШЫцЪБМфБфЛЏЧщПіШчЭМЫљЪОЁЃ
2NO2(g) ІЄHЃНЃЋ57.0kJЁЄmolЃ1ЃЌNO2КЭN2O4ЕФХЈЖШЫцЪБМфБфЛЏЧщПіШчЭМЫљЪОЁЃ

ЃЈ3ЃЉдк0~60sФкЃЌвдN2O4БэЪОЕФЦНОљЗДгІЫйТЪЮЊ__________molЁЄLЃ1ЁЄsЃ1ЁЃ
ЃЈ4ЃЉИљОнЭМжагаЙиЪ§ОнЃЌМЦЫу100ЁцЪБИУЗДгІЕФЦНКтГЃЪ§K1ЃН__________ЁЃШєЦфЫћЬѕМўВЛБфЃЌЩ§ИпЮТЖШжС120ЁцЃЌДяЕНаТЦНКтЪБЕФЦНКтГЃЪ§ЪЧK2ЃЌдђK1_____K2(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁАЃНЁБ)ЁЃ
ЃЈIIIЃЉЯђШнЛ§ЮЊ2LЕФУмБеШнЦїжаЭЈШывЛЖЈСПЕФCOКЭH2OЃЌЗЂЩњЗДгІЃКCO(g)ЃЋH2O(g) ![]() H2(g)ЃЋCO2(g)ЁЃ
H2(g)ЃЋCO2(g)ЁЃ
ЃЈ5ЃЉЯТСаЫЕЗЈФмзїЮЊХаЖЯИУЗДгІДяЕНЛЏбЇЦНКтзДЬЌЕФвРОнЕФЪЧ____(ЬюзжФИађКХ)ЁЃ
A.ШнЦїФкCOЁЂH2OЁЂCO2ЁЂH2ЕФХЈЖШжЎБШЮЊ1ЃК1ЃК1ЃК1
B.COЕФЯћКФЫйТЪгыH2ЕФЯћКФЫйТЪЯрЕШ
C.ШнЦїФкбЙЧПБЃГжВЛБф
D.ЛьКЯЦјЬхЕФУмЖШБЃГжВЛБф
ЃЈ6ЃЉБЃГжЦфЫћЬѕМўВЛБфЃКдкVLУмБеШнЦїжаЭЈШы10molCOКЭ10molH2O(g)ЗЂЩњЩЯЪіЗДгІЃЌдкTЁцДяЕНЦНКтЃЌШЛКѓМБЫйГ§ШЅЫЎеєЦј(Г§ЫЎеєЦјЪБЦфЫћИїГЩЗжЕФЮяжЪЕФСПВЛБф)ЃЌНЋЛьКЯЦјЬхШМЩеЃЌВтЕУЗХГіЕФШШСПЮЊ2842kJ(вбжЊCOЕФШМЩеШШЮЊ283kJЁЄmolЃ1ЃЌH2ЕФШМЩеШШЮЊ286kJЁЄmolЃ1)ЃЌдђTЁцЦНКтГЃЪ§KЃН______ЁЃЃЈОЋШЗЕНаЁЪ§ЕуКѓСНЮЛЃЉ
ЁОД№АИЁП2ІЄH1+ ІЄH2 H2S(g)+2O2(g) =SO42-(aq)+2H+(aq) ІЄH =Ѓ806.39 kJЁЄmol-1 1ЁС10-3 0.36 < B 0.44
ЁОНтЮіЁП
ЃЈ1ЃЉРћгУИЧЫЙЖЈТЩМЦЫуЃЛ
ЃЈ2ЃЉИљОнЭМЯёаДГіСНВНЕФШШЛЏбЇЗНГЬЪНЃЌдйИљОнИЧЫЙЖЈТЩМДЕУЃЛ
ЃЈ3ЃЉИљОнv=![]() МЦЫуЃЛ
МЦЫуЃЛ
ЃЈ4ЃЉИљОнЦНКтГЃЪ§ЕФБэДяЪНКЭЭМЯёМЦЫуЃЛвђЮЊИУЗДгІЕФьЪБфЁїH>0ЃЌЩ§ИпЮТЖШЃЌkдіДѓЃЛ
ЃЈ5ЃЉИљОнЦНКтКѓе§ФцЗДгІЫйТЪЯрЕШЃЌБфСПВЛдкЗЂЩњИФБфЗжЮіЃЛ
ЃЈ6ЃЉИљОнЗХГіЕФШШСПМЦЫуГіCOКЭЧтЦјЕФЮяжЪЕФСПЃЌдкМЦЫуЦНКтГЃЪ§ЁЃ
ЃЈ1ЃЉвбжЊЃКЂйH2S(g) ![]() H2(g)ЃЋS(g) ІЄH1ЃЛ Ђк2S(g)
H2(g)ЃЋS(g) ІЄH1ЃЛ Ђк2S(g) ![]() S2(g) ІЄH2ЃЌИљОнИЧЫЙЖЈТЩЂйЁС2+ЂкЕУ2H2S(g)
S2(g) ІЄH2ЃЌИљОнИЧЫЙЖЈТЩЂйЁС2+ЂкЕУ2H2S(g) ![]() 2H2(g)ЃЋS2(g) ІЄHЃН2ІЄH1+ ІЄH2ЃЛ
2H2(g)ЃЋS2(g) ІЄHЃН2ІЄH1+ ІЄH2ЃЛ
Д№АИЃК2ІЄH1+ ІЄH2ЃЛ
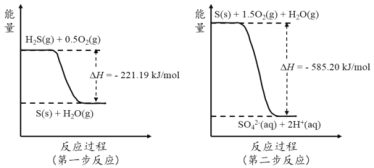
ЃЈ2ЃЉгЩЭМПЩжЊЃЌЕквЛВНШШЛЏбЇЗДгІЮЊЃКH2SЃЈgЃЉ+0.5O2ЃЈgЃЉ=SЃЈsЃЉ+H2OЃЈgЃЉЁїH=Ѓ221.19 kJmol-1ЃЛЕкЖўВНЗДгІЮЊЃКSЃЈsЃЉ+1.5O2ЃЈgЃЉ+H2OЃЈgЃЉ=2H+ЃЈaqЃЉ+SO42-ЃЈaqЃЉЃЉЁїH=-585.20 kJmol-1ЃЛвРОнИЧЫЙЖЈТЩЃЌЕквЛВНгыЕкЖўВНЗНГЬЪНЯрМгЕУЃКH2S(g)+2O2(g) =SO42-(aq)+2H+(aq) ІЄH =Ѓ806.39 kJЁЄmol-1ЃЛ
Д№АИЃКH2S(g)+2O2(g) =SO42-(aq)+2H+(aq) ІЄH =Ѓ806.39 kJЁЄmol-1ЃЛ
ЃЈ3ЃЉгЩМзЭМПЩжЊЃЌдк60sЪБЃЌN2O4ЕФХЈЖШЮЊ0.04 molЁЄL-1ЃЌЫљвдN2O4БэЪОЕФЦНОљЗДгІЫйТЪЮЊv=(0.1-0.04) molЁЄL-1ЁТ60s=1ЁС10-3 molЁЄL-1ЁЄs-1ЃЛ
Д№АИЃК1ЁС10-3ЃЛ
ЃЈ4ЃЉМзЭМПЩжЊдкЗДгІЕН60sЪБЃЌЗДгІЮяКЭЩњГЩЮяЕФХЈЖШБЃГжВЛБфЃЌЫљвдДЫЪБЗДгІДяЦНКтЃЌЦНКтГЃЪ§K1=![]() =
=![]() =0.36mol.L-1.s-1ЃЛвђЮЊИУЗДгІЕФьЪБфЁїH>0ЃЌЫљвдЪЧИіЮќШШЗДгІЃЌЫљвдЩ§ИпЮТЖШЃЌЦНКте§ЯђвЦЖЏЃЌЦНКтГЃЪ§діДѓЃЌk1<k2ЃЛ
=0.36mol.L-1.s-1ЃЛвђЮЊИУЗДгІЕФьЪБфЁїH>0ЃЌЫљвдЪЧИіЮќШШЗДгІЃЌЫљвдЩ§ИпЮТЖШЃЌЦНКте§ЯђвЦЖЏЃЌЦНКтГЃЪ§діДѓЃЌk1<k2ЃЛ
Д№АИЃК0.36ЃЛ<ЃЛ
ЃЈ5ЃЉA.ШнЦїФкCOЁЂH2OЁЂCO2ЁЂH2ЕФХЈЖШжЎБШЮЊ1ЃК1ЃК1ЃК1ЪБЃЌЗДгІВЛвЛЖЈДяЕНСЫЦНКтЃЌЙЪAДэЮѓЃЛ
B.COЕФЯћКФЫйТЪДњБэе§ЗДгІЫйТЪЃЌH2ЕФЯћКФЫйТЪДњБэФцЗДгІЫйТЪЃЌЖўепЫйТЪБШЕШгкЛЏбЇМЦСПЪ§жЎБШЃЌвђДЫЗДгІДяЕНСЫЦНКтЃЌЙЪBе§ШЗЃЛ
C.вђЮЊЗДгІЧАКѓЦјЬхЕФзмЮяжЪЕФСПЪМжеВЛБфЃЌЙЪШнЦїФкбЙЧПЗДгІжаЪМжеВЛБфЃЌВЛФмХаЖЯЪЧЗёДяЕНЦНКтЃЌЙЪCДэЮѓЃЛ
D.ЗДгІЙ§ГЬжаЃЌЛьКЯЦјЬхЕФУмЖШЪМжеБЃГжВЛБфЃЌВЛФмХаЖЯЪЧЗёДяЕНЦНКтЃЌЙЪDДэЮѓЃЛ
Д№АИЃКBЃЛ
ЃЈ6ЃЉИљОнЗНГЬЪНЕФЯЕЪ§ПЩвдПДГіЃЌШчЙћМйЩшЩњГЩЧтЦјЮЊxmolЃЌдђвЛбѕЛЏЬМЪЃгрЃЈ10-xЃЉmolЃЌИљОнШМЩеЗХГіЕФШШСПСаГіЗНГЬЃК286x+283ЃЈ10-xЃЉ=2842ЃЌНтЕУx=4ЃЌЫљвдДяЕНЦНКтЪБЃЌвЛбѕЛЏЬМЁЂЫЎеєЦјЕФЮяжЪЕФСПЗжБ№ЮЊ6molЃЌЧтЦјЁЂЖўбѕЛЏЬМЕФЮяжЪЕФСПЗжБ№ЮЊ4molЁЃгжгЩгкЗДгІЧАКѓЦјЬхЛЏбЇМЦСПЪ§ЯрЕШЃЌдкЦНКтГЃЪ§ЕФБэДяЪНжаЃЌШнЦїЕФЬхЛ§ПЩвддМШЅЃЌk=![]() =
=![]() =0.44ЁЃ
=0.44ЁЃ
Д№АИЃК0.44ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвдЕЊЛЏяи(GaN)ЁЂЩщЛЏяи(GaAs)ЮЊДњБэЕФЕкШ§ДњАыЕМЬхВФСЯФПЧАвбГЩЮЊШЋЧђАыЕМЬхбаОПЕФЧАбиКЭШШЕуЃЌШчЩщЛЏяиЕЦХнЪйУќЪЧЦеЭЈЕЦХнЕФ100БЖЃЌЖјКФФмНіЮЊ10%ЃЌЭЦЙуЩщЛЏяиЕШЗЂЙтЖўМЋЙм(LED)ееУїЃЌЪЧНкФмМѕХХЕФгааЇОйДыЁЃЧыЛиД№ЯТСаЮЪЬтЃК
(1)яиЮЊдЊЫижмЦкБэЕк31КХдЊЫиЃЌЛљЬЌяидзгЕФЕчзгХХВМЪНЮЊ___________ЃЌКЫЭтЕчзгеМОнзюИпФмВуЗћКХЮЊ________ЁЃ
(2)ЕЊЛЏяигыН№ИеЪЏОпгаЯрЫЦЕФОЇЬхНсЙЙЃЌЕЊЛЏяижаЕЊдзггыяидзгжЎМфвд_______МќЯрНсКЯЃЌЕЊЛЏяиЪєгк_______ОЇЬхЁЃ
(3)ЯТСаЫЕЗЈе§ШЗЕФЪЧ_______
A.ЕквЛЕчРыФмЃКAs < Ga B.ЩщКЭяиЖМЪєгкpЧјдЊЫи
C.ЕчИКадЃКAs < GaЁЁ D.АыЕМЬхGaPЁЂSiCгыЩщЛЏяиЮЊЕШЕчзгЬх
(4)ЂйЩщЛЏяиЪЧНЋ(CH3)3GaКЭAsH3гУMOCVD(Н№ЪєгаЛњЮяЛЏбЇЦјЯрЕэЛ§)ЗНЗЈжЦБИЕУЕНЕФЃЌИУЗДгІдк700ЁцНјааЃЌЗДгІЕФЗНГЬЪНЮЊЃК____________________ЁЃ
ЂкЗДгІЮяAsH3ЗжзгЕФМИКЮЙЙаЭЮЊ_______ЃЌ(CH3)3GaжаяидзгдгЛЏЗНЪНЮЊ___ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЃКгаЛњЮяAЕФВњСППЩвдгУРДКтСПвЛИіЙњМвЕФЪЏгЭЛЏЙЄЗЂеЙЫЎЦНЁЃЯжвдAЮЊжївЊдСЯКЯГЩввЫсввѕЅЃЌЦфКЯГЩТЗЯпШчЭМ1ЫљЪОЁЃ

ЃЈ1ЃЉBЗжзгжаЙйФмЭХЕФУћГЦЪЧ________ЃЌDжаЙйФмЭХЕФУћГЦЪЧ_________ЃЛЗДгІЂйЕФЗДгІРраЭЪЧ__________ЗДгІЁЃ
ЃЈ2ЃЉЗДгІЂкЕФЛЏбЇЗНГЬЪНЪЧ________________________________________ЃЌЗДгІЂнЕФЛЏбЇЗНГЬЪНЪЧ_________________________________________________ЁЃ
ЃЈ3ЃЉЂйФГЭЌбЇгУШчЭМ2ЫљЪОЕФЪЕбщзАжУжЦШЁЩйСПввЫсввѕЅЃЎЪЕбщНсЪјКѓЃЌЪдЙмМзжаЩЯВуЮЊЭИУїЕФЁЂВЛШмгкЫЎЕФгЭзДвКЬхЁЃЩЯЪіЪЕбщжаЪдЙмМзжаЪдМСЮЊ___________________ЃЌЦфзїгУЪЧ(ЬюзжФИ)_____________ЁЃ
AЃЎжаКЭввЫсКЭввДМ
BЃЎжаКЭввЫсВЂЮќЪеВПЗжввДМ
CЃЎввЫсввѕЅдкБЅКЭЬМЫсФЦШмвКжаЕФШмНтЖШБШдкЫЎжаИќаЁЃЌгаРћгкЗжВуЮіГі
DЃЎМгЫйѕЅЕФЩњГЩЃЌЬсИпЦфВњТЪ
ЂкдкЪЕбщЪвРћгУBКЭDжЦБИввЫсввѕЅЕФЪЕбщжаЃЌШєгУ1mol BКЭ1mol DГфЗжЗДгІЃЌ_____(Фм/ВЛФм)ЩњГЩ1mol ввЫсввѕЅЃЌдвђЪЧ_____________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙигкЯТСаЭМЯѓЫЕЗЈе§ШЗЕФЪЧ
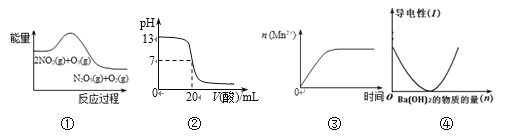
A. ЂйБэЪОЛЏбЇЗДгІ2NO2(g) + O3(g) = N2O5(g) + O2(g) ІЄH > 0
B. ЂкБэЪО25ЁцЪБЃЌгУ0.1 mol/L CH3COOHШмвКЕЮЖЈ20 mL 0.1 mol/L NaOHШмвКЃЌШмвКЕФpHЫцМгШыЫсЬхЛ§ЕФБфЛЏ
C. ЂлБэЪО10 mL 0.01 mol/LЫсадKMnO4ШмвКгыЙ§СПЕФ0.1 mol/L H2C2O4ШмвКЛьКЯЪБЃЌ n (Mn2+) ЫцЪБМфЕФБфЛЏ
D. ЂмПЩБэЪОЯђЯЁСђЫсШмвКжаЕЮМгЧтбѕЛЏБЕШмвКЃЌШмвКЕМЕчадЫцЧтбѕЛЏБЕЮяжЪЕФСПЕФБфЛЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭУЖњВнШЉHЪЧвЛжжживЊЕФЯуСЯЃЌжївЊгУгкЪГЦЗЁЂЛЏзБЦЗЕШЙЄвЕжаЁЃгУгаЛњЮяAЮЊдСЯПЩвдКЯГЩЭУЖњВнШЉHЃЌЦфКЯГЩТЗЯпШчЭМЫљЪОЃК
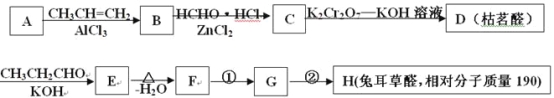
жаМфВњЮяDЪЧвЛжжОЋЯИЛЏЙЄВњЦЗЃЌПЩгУзїЯуСЯЃЌФмЗЂЩњШчЯТЗДгІЃК
вбжЊЃКЂёЃЎвбжЊЃКШЉгыЖўдЊДМ(ШчввЖўДМ)ПЩЩњГЩЛЗзДЫѕШЉЃК
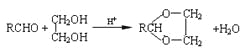

ЧыЛиД№ЃК
ЃЈ1ЃЉDЕФНсЙЙМђЪНЮЊ____________ЃЌEжаКЌгаЕФЙйФмЭХУћГЦЮЊ_____________ЁЃ
ЃЈ2ЃЉAЗжзгжаЬМЁЂЧтЕФжЪСПБШЮЊ12ЉU1ЃЌAЕФЗжзгЪНЮЊ_____________ЃЌBЕФНсЙЙМђЪНЮЊ__________________________________ЁЃ
ЃЈ3ЃЉЗДгІЂйЕФЗДгІРраЭ____________________________, ЗДгІЂкЕФЛЏбЇЗНГЬЪНЮЊ___________________________ЁЃ
ЃЈ4ЃЉЭУЖњВнШЉHжаЕФКЌбѕЙйФмЭХвзБЛбѕЛЏЃЌЩњГЩЛЏКЯЮяWЃЌ GгыWПЩЗЂЩњѕЅЛЏЗДгІЃЌаДГіGгыWЗДгІЕФЛЏбЇЗНГЬЪН_____________________________________________
ЃЈ5ЃЉWгы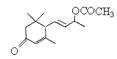 ЪЧЗёЛЅЮЊЭЌЗжвьЙЙЬх_____ЃЈЬюЁАЪЧЁБЛђЁАЗёЁБЃЉЃЌЗћКЯЯТСаЬѕМўЕФ
ЪЧЗёЛЅЮЊЭЌЗжвьЙЙЬх_____ЃЈЬюЁАЪЧЁБЛђЁАЗёЁБЃЉЃЌЗћКЯЯТСаЬѕМўЕФ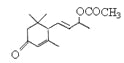 ЕФЭЌЗжвьЙЙЬхга__________жжЃЌаДГіЦфжавЛжжЕФНсЙЙМђЪН_________________________ЁЃ
ЕФЭЌЗжвьЙЙЬхга__________жжЃЌаДГіЦфжавЛжжЕФНсЙЙМђЪН_________________________ЁЃ
aЃЎЪєгкЗМЯузхЛЏКЯЮяЧвБНЛЗЩЯгаЮхИіШЁДњЛљ
bЃЎКЫДХЙВеёЧтЦзгаЫФжжРраЭЧтдзгЕФЮќЪеЗх
cЃЎ1molИУЮяжЪзюЖрПЩЯћКФ2molNaOH
dЃЎФмЗЂЩњвјОЕЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
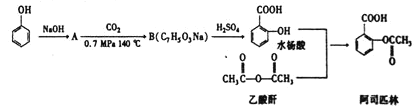
ЁОЬтФПЁПЗЧХЕБДЬи(fenofibrate)ЪЧНЕЕЈЭЌДММАИЪгЭШ§ѕЅЕФвЉЮяЃЌЫќЕФвЛЬѕКЯГЩТЗЯпШчЯТЃК
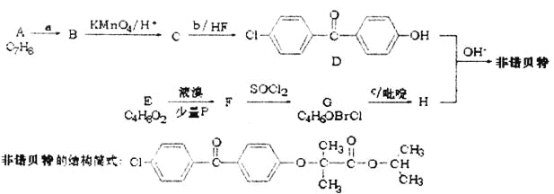
вбжЊЃКЂйєШЫсРргаЛњЮягывКфхдкЩйСПСззїгУЯТЃЌЗЂЩњ![]() ШЁДњЁЃ
ШЁДњЁЃ
![]()
ЃЈ1ЃЉBЕФУћГЦЮЊ_______________ЁЃ
ЃЈ2ЃЉCЫљКЌЙйФмЭХЕФУћГЦЮЊ___________ЁЃ
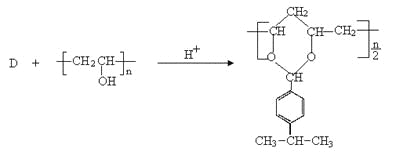
ЃЈ3ЃЉаДГіЯТСаЮяжЪЕФНсЙЙМђЪНb_____________ЃЌF__________________ЁЃ
ЃЈ4ЃЉаДГіGЕНHЕФЗДгІЗНГЬЪН_______________ЁЃ
ЃЈ5ЃЉаДГіЭЌЪБЗћКЯЯТСаЬѕМўЕФDЕФЭЌЗжвьЙЙЬхНсЙЙЭВЪН____________ЁЃ
ЂйФмЗЂЩњвјОЕЗДгІЃЛЂкКЌ5жжВЛЭЌЛЗОГЧтЕФСЊБНѕЅРргаЛњЮяЁЃ
1 molИУгаЛњЮязюЖрЯћКФNaOHЕФЮяжЪЕФСПЮЊ_______________ЁЃ
ЃЈ6ЃЉвд2-МзЛљБћЯЉЮЊдСЯжЦБИEЃЌЩшМЦКЯГЩТЗЯп(ЦфЫћЪдМСШЮбЁ)ЁЃ
___________________________________________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПН№ЪєюбадФмгХдНЃЌБЛГЦЮЊМЬЬњЁЂТСжЎКѓЕФЁАЕкШ§Н№ЪєЁБЁЃюбЬњПѓ(жївЊГЩЗжЮЊFeTiO3ЃЌКЌЩйСПFe2O3ЁЂSiO2ЕШдгжЪ)ПЩгУРДжЦБИTiO2ЃЌЭЌЪБЕУЕНИБВњЦЗТЬЗЏ(FeSO4ЁЄ7H2O)ЃЌЙЄвеСїГЬШчЭМЫљЪОЃК

вбжЊЃКЂйFeTiO3ЃЋ2H2SO4ЃНFeSO4ЃЋTiOSO4ЃЋ2H2O
ЂкTiO2ЃЋвзЫЎНтЃЌжЛФмДцдкгкЧПЫсадШмвКжа
ЃЈ1ЃЉЫсНўIжаFe2O3гыЯЁСђЫсЗДгІЕФРызгЗНГЬЪНЃК_____________________ЁЃ
ЃЈ2ЃЉЙ§ГЬIIжаМгШыЪЪСПЬњаМЕФФПЕФЪЧ_____________________________ЁЃ
ЃЈ3ЃЉЗжРыIIIжаВНжшЂкЕУЕНТЬЗЏЕФВйзїЪЧ__________________________ЁЃ
ЃЈ4ЃЉгЩТЫвКIVЬсШЁTiO2ЕФЙ§ГЬШчЯТЃК

ЂйЧыгУЛЏбЇЦНКтвЦЖЏдРэНтЪЭТЫвКМгШШжѓЗаЕФФПЕФЃК_______________ЁЃ
ЂкгЩ2MgЃЋTiCl4ЁњTiЃЋ2MgCl2ЗДгІКѓЕУЕНMgЁЂMgCl2ЁЂTiЕФЛьКЯЮяЃЌПЩВЩгУецПееєСѓЕФЗНЗЈЗжРыЕУЕНTiЃЌвРОнШчБэаХЯЂЃЌашМгШШЕФЮТЖШТдИпгк______ЁцМДПЩЁЃ
TiCl4 | Mg | MgCl2 | Ti | |
ШлЕу/Ёц | -26.0 | 648.8 | 714 | 1667 |
ЗаЕу/Ёц | 136.4 | 1090 | 1412 | 3287 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭМ1ЪЧЭаПдЕчГиЪОвтЭМЁЃЭМ2жаЃЌxжсБэЪОЪЕбщЪБСїШые§МЋЕФЕчзгЕФЮяжЪЕФСПЃЌyжсБэЪОЃЈЁЁЁЁЃЉ
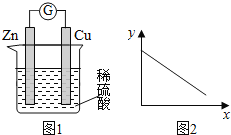
A. ЭАєЕФжЪСП B. c(Zn2ЃЋ) C. c(HЃЋ) D. c(SO42-) -
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАЂЫОЦЅСжвВНаввѕЃЫЎбюЫсЃЌЪЧАйФъРДШ§ДѓОЕфвЉЮяжЎвЛЃЎгУгкжЮИаУАЁЂЗЂШШЁЂЭЗЭДЁЂбРЭДЁЂЙиНкЭДЁЂЗчЪЊВЁЃЌЛЙФмвжжЦбЊаЁАхОлМЏЃЌгУгкдЄЗРКЭжЮСЦШБбЊадаФдрВЁЁЂаФНЪЭДЁЂаФЗЮЙЃШћЁЂФдбЊЫЈаЮГЩЃЌвВПЩгІгУгкбЊЙмаЮГЩЪѕМАХдТЗвЦжВЪѕЃЎАЂЫОЦЅСжОЕфЕФКЯГЩЗНЗЈШчЯТСїГЬЃК
ЛиД№ЯТСаЮЪю}ЃК
(1)АЂЫОЦЅСжЕФЗжзгЪНЮЊ ______ ЃЌЗжзгжаЫљКЌЙйФмЭХЮЊ ______ ЁЂ ______ ЃЛЫЎбюЫсЕФЯЕЭГУќУћЮЊ ______ЁЃ
(2)ЫЎбюЫсКЭввЫсєћЩњГЩАЂЫОЦЅСжЕФЗДгІРраЭЪєгк ______ ЃЌЗДгІЙ§ГЬжаПижЦЮТЖШдк![]() ЃЌШєЮТЖШЙ§ИпвзЗЂЩњЦкЗДгІЃЌПЩФмЩњГЩЕФИБВњЮяжаЪєгкжЌЕФЮЊ ______
ЃЌШєЮТЖШЙ§ИпвзЗЂЩњЦкЗДгІЃЌПЩФмЩњГЩЕФИБВњЮяжаЪєгкжЌЕФЮЊ ______ ![]() аДГіСНжжЮяжЪЕФНсЙЙМђЪНЃЌАИіЗжзгжажЛгаСНИіЛЗ
аДГіСНжжЮяжЪЕФНсЙЙМђЪНЃЌАИіЗжзгжажЛгаСНИіЛЗ![]() ЁЃ
ЁЃ
(3)![]() ЩњГЩBЕФЗДгІЗНГЬЪНЮЊ ______ ЃЌАЂЫОЦЅСжгызуСПЕФNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ______ ЁЃ
ЩњГЩBЕФЗДгІЗНГЬЪНЮЊ ______ ЃЌАЂЫОЦЅСжгызуСПЕФNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ______ ЁЃ
(4)ЫЎбюЫсгаЖржжЭЌЗжвьЙЙЬхЃЌЗћКЯЯТСаЬѕМўЕФЗМЯузхЛЏКЯЮяга ______ жжЁЃ
ЂйФмЗЂЩњвјОЕЗДгІЂкШ§ТШЛЏЬњШмвКГЪзЯЩЋЁЃ
(5)БНЗгвВгаЖржжЭЌЗжвьЙЙЬхЃЌаДГіЦфКЫДХЙВеёЧтЦзжЛгавЛжжЕФЮяжЪЕФНсЙЙМђЪН ____ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com